Sandbox Reserved 962
From Proteopedia
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
mRNA Cap (Guanine-N7) Methyltransferase (Ecm1)
|
Contents |
Biological role
Structure
Interaction
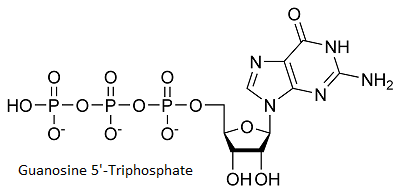
Cap Analog Binding (Guanosine 5'-Triphosphate)
binds to the enzyme in a pocket near AdoHcy. (Tyr 145, Leu216, Leu217, Asp218, Ser219, Tyr284) are involded in the binding of the cap analog, more precisely they interact with guanine and with the guanine exocyclic 2-NH2. The cap makes Van der Walls contacts with side chains fromGTP makes a hydrogen bond with and a water mediated bond with . [1]
As we can see on the figure above[2] , the enzyme specifically binds to guanine. This specificity is achieved through different recognitions. The N-1 atom of adenine is unprotonated, this prevent the interaction of adenine with Ecm1. Ecm1 contact the O6 atom of guanine and permit an additional discrimination between guanine and adenine. Moreover the fact that ITP is not a substrate for Ecm1 show that the interactions between Ecm1 and guanine exocyclic 2-NH2 are important for substrate binding.We also remark that the methyltransferase is not able to discrminate between ribose and desoxyribose nucleoside sugars.
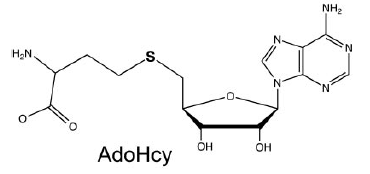
AdoHcy Binding (S-Adenosyl-L-Homocysteine)
The mRNA Cap Methyltransferase bind to which is the product of the methyl donor AdoMet after the methylation. AdoHcys is in a pocket formed by amino acids of segment 2.(Lys54, Gly72, Asp78, Asp94, Ile95, Asp122, Ser124, Gln140, Phe141, Ser142) are involved in the stabilisation of AdoHcys.
The interactions between AdoHcys and the enzyme are made of : - Hydrogen bonds mediated by - Van der Walls interactions mediated by - An electrostatic interaction mediated by- A water mediated contact mediated by [1]
Mechanism
This enzyme catalyse N-methyl transfer from AdoMet (S-adenosylmethionine) to GpppRNA, this reaction produce 7-methyl-GpppRNA and AdoHcy. This reaction is made through a SN2 mechanism. We remark that there is no contact between the enzyme and or the AdoMet methyl carbon.Indeed the enzyme does not stabilize the transition state of the chemical reaction, does not promote the activation of the nucleophile or the expulsion of the leaving group. mRNA Cap Methyltransferase brings the two substrates closer and orientates the substrates to facilitate the methyl transfer.
Inhibition
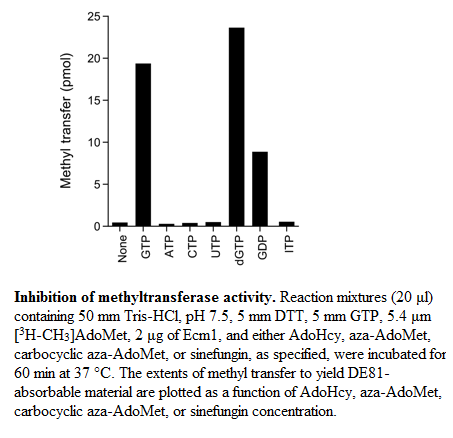
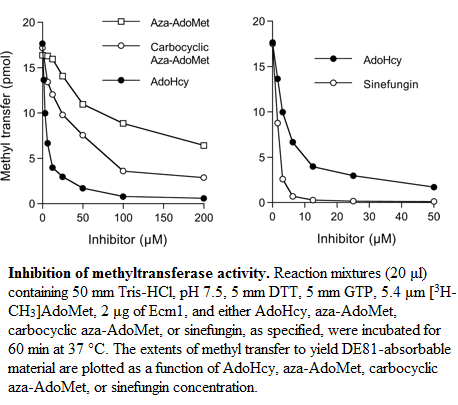 It was show that the methylation of GTP increase with the concentration of AdoMet (Km of 25μM) and it was determined that the product AdoHcy has a similar affinity than AdoMet. But the activity of Ecm1 is inhibited by AdoHcy in a concentration-dependant way and the apparent IC50 was 4μm.
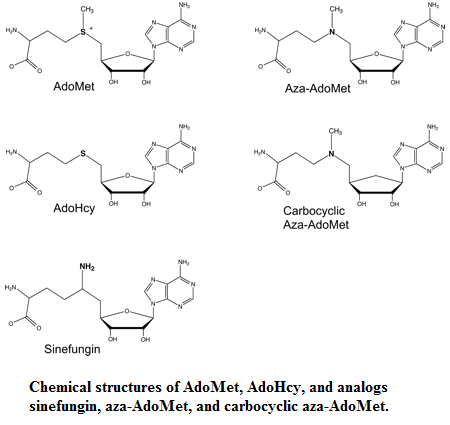
Sinefugin, an analog of AdoMet differs from AdoMet only in the S-CH3 which is replaced by a C-NH2. It was show that sinefugin inhibit Ecm1 in a concentration-dependant manner too, the apparent IC50 is 1.5μm. The Ecm1 has an affinity for sinefugin 2/3 fold higher than for AdoMet and AdoHcy. Sinefugin has been shown to have antifungal, antiprotozoal and antiviral activities, these activities is probably related to his capacity to inhibit a variety of AdoMet-dependent methyltransferase.
It was show that the methylation of GTP increase with the concentration of AdoMet (Km of 25μM) and it was determined that the product AdoHcy has a similar affinity than AdoMet. But the activity of Ecm1 is inhibited by AdoHcy in a concentration-dependant way and the apparent IC50 was 4μm.
Sinefugin, an analog of AdoMet differs from AdoMet only in the S-CH3 which is replaced by a C-NH2. It was show that sinefugin inhibit Ecm1 in a concentration-dependant manner too, the apparent IC50 is 1.5μm. The Ecm1 has an affinity for sinefugin 2/3 fold higher than for AdoMet and AdoHcy. Sinefugin has been shown to have antifungal, antiprotozoal and antiviral activities, these activities is probably related to his capacity to inhibit a variety of AdoMet-dependent methyltransferase.
Aza-AdoMet and carbocyclic aza-AdoMet are analogs of AdoMet too. In these two molecules the sulfur atom is replaced by nitrogen. And in the carbocyclic derivate the O4-atom of the ribose is replaced by a methylene group. These two molecules are weak inhibitorsof Ecm1. The IC50 values of Aza-AdoMet is 100μm and of carbocyclic aza-AdoMet is 35μm
References
- ↑ 1.0 1.1 Fabrega C, Hausmann S, Shen V, Shuman S, Lima CD. Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol Cell. 2004 Jan 16;13(1):77-89. PMID:14731396
- ↑ Hausmann S, Zheng S, Fabrega C, Schneller SW, Lima CD, Shuman S. Encephalitozoon cuniculi mRNA cap (guanine N-7) methyltransferase: methyl acceptor specificity, inhibition BY S-adenosylmethionine analogs, and structure-guided mutational analysis. J Biol Chem. 2005 May 27;280(21):20404-12. Epub 2005 Mar 9. PMID:15760890 doi:10.1074/jbc.M501073200