Mycobacterium tuberculosis ArfA Rv0899
From Proteopedia
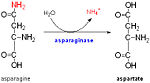
FunctionProtein Rv0899 from Mycobacterium tuberculosis [1] belongs to the OmpA (outer membrane protein A) family of outer membrane proteins.The deletion of this gene impairs the uptake of some water-soluble substances, such as serine, glucose, and glycerol.Using NMR chemical shift perturbation and isothermal calorimetric titration assays, Rv0899 was able to interact with , which may indicate a role for Rv0899 in the process of Zn(2+) acquisition. [1] Mycobacterium tuberculosis ArfA (Rv0899) is a membrane protein encoded by an ammonia release facilator operon that is necessary for rapid ammonia secretion, pH neutralization and adaptation to acidic environments in vitro. Its C-terminal domain (C domain) shares significant sequence homology with the OmpA-like family of peptidoglycan-binding domains, suggesting that its physiological function in acid stress protection may be related to its interaction with the mycobacterial cell wall. It exhibits pH-dependent conformational dynamics (with significant heterogeneity at neutral pH and a more ordered structure at acidic pH), which could be related to its acid stress response. The C domain associates tightly with polymeric peptidoglycan isolated from Mycobacterium tuberculosis. Its functions in acid stress protection and peptidoglycan binding suggest a link between the acid stress response and the physicochemical properties of the mycobacterial cell wall.[2]. Structure SectionThe 326-residue protein contains three domains: an N-terminal domain (residues 1-72) DiseaseTuberculosis (TB) is the seventh most common cause of death globally (1). Mycobacterium tuberculosis, its causative agent, has achieved a spread in the human population unmatched by any other bacterial pathogen (2), despite the availability of effective short-course chemotherapy and the Bacille Calmette-Guerin vaccine. Important factors contributing to the high pathogenicity of M. tuberculosis are its highly impermeable cell wall, which prevents the Rv0899 has been proposed to act as an outer membrane porin and to contribute to the bacterium's adaptation to the acidic environment of the phagosome[3] during infection.uptake of most hydrophilic antibiotic drugs, and its high adaptability to environmental changes during the course of infection, including: nutrient deprivation, hypoxia, various exogenous stress conditions and the phagosomal environment (3). The complete genome sequence of M. tuberculosis H37Rv, the best-characterized strain of the bacterium, has identified several stress response genes which contribute to pathogenicity (4). Among these, the membrane protein Rv0899, encoded by the rv0899 gene, is thought to confer adaptation of M. tuberculosis to the acidic environment of the phagosome (5). Sequence analysis of the mycobacterial genomes shows that the gene is restricted to pathogenic mycobacteria associated with TB (M. tuberculosis, M. bovis) and other TB-related diseases (M. marinum, M. ulcerans, M. kansaii) and, thus, is an attractive candidate for the development of anti-TB chemotherapeutic agents. Notably, two M. tuberculosis H37Rv genes (Rv0900 and Rv0901) adjacent to Rv0899 also encode putative membrane proteins, and are found exclusively in association with Rv0899 in the same pathogenic mycobacteria, suggesting that the three may constitute an operon dedicated to a common function The amino acid pair Asn111-Gly112,located at the end of α1 and preceding L3, undergoes in-vitro deamidation, a pH-dependent reaction whereby Asn is converted to Asp and ammonia is released. Asparagine residues preceding glycine, and situated in conformationally flexible regions of proteins, are frequently deamidated, with potentially significant consequences for protein regulation and function (47). In the case of Rv0899, deamidation and the concomitant release of ammonia could have important consequences for the acid adaptation function of the protein. The gene is restricted to pathogenic mycobacteria and, thus, is an attractive candidate for the development of anti-tuberculosis chemotherapy. [3] Probably plays a role in ammonia secretion that neutralizes the medium at pH 5.5,and preceded exponential growth of Mycobacterium tuberculosis, although it does not play a direct role in ammonia transport.[ARFA_MYCTU]. The ompATb operon is necessary for rapid ammonia secretion and adaptation of M. tuberculosis to acidic environments in vitro but not in mice. [4] RelevanceStructural highlights
References
| ||||||||||||||||