Antifreeze proteins (AFPs) evolved in various organisms permitting their survival in subzero environments[1]. They exhibit remarkable structural diversity and molar activities across the various kingdoms[2]. The longhorn beetle, Rhagium inquisitor, has the ability to supercool to below -25 °C partially due to the presence of a highly potent AFP (RiAFP) in its hemolymph. RiAFP is a 13-kDa protein with one of the highest antifreeze activities measured for any AFP.
Function
RiAFP, like other AFPs, adsorb to the surface of ice crystals and lower the temperature at which these crystals grow. Consequently, creating a difference between the melting point and the freezing point known as thermal hysteresis (TH), within which the ice growth is arrested[3]. It is well accepted that AFPs inhibit ice crystals growth through the adsorption inhibition model[4]. According to this model AFPs bind to an ice crystal surface and block water molecules from accessing it at the bound location. The ice front thus becomes convex toward the solution between the surface-bound AFPs, creating the microcurvature. Thus, the ice grow is less favorable due to Gibbs-Thompson-Herring (Kelvin) effect, which leads to noncolligative depression of the freezing point (Tf) of the solution (thermal hysteresis).
Overall Structure
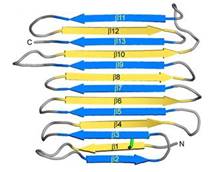
Fig. 1. Secondary structure diagram
The crystallographic structure of RiAFP was defined recently
[5]. RiAFP has a novel β-solenoid architecture that forms . This sandwich is composed of two parallel remarkably regular . The β-sheets lie on top of each other with the upper and lower strands parallel but in the opposite orientation. β1 and β2 strands are untiparallel to the other strands in each sandwich plane. Two ends deviate from β helix regularity by forming (see Figure 1). These capping structures help to prevent end-to-end associations that would spoil the solubility of RiAFP and lead to oligomerization and aggregation.
The three residues in β-strand 11 at the C terminus that are too bulky to be accommodated into the core may also contribute to the capping structure to prevent amyloid-like polymerization. RiAFP solenoid possesses compressed nature with average distance between the sheets of only 6 Å. In the core of RiAFP, the side chains within apposed β-strands from the two β-sheets are staggered, allowing the side chains to interdigitate and pack tightly against one another. Most of the in the core are from Ala, Ser and Thr. Those residues create a more compact fold that may contribute to the high stability and antifreeze activity of RiAFP. Within the core there are between Thr-Ser (65-55, 85-75, 132-124 respectively) and one between Cys4-Cys21, that contributes to stabilization of the whole structure. The β-turns in the structure contain mostly Gly or Pro residues.
The asymmetric unit comprises two RiAFP molecules juxtaposed with their ice-binding surfaces, however the protein is monomer in the solution.
Ice Binding Surface (IBS)
IBS of RiAFP contains five expanded within the top β–sheet. These motifs are remarkably regular, allowing any rows/columns of TXTXTXT motifs to be exactly superposed onto any other rows/columns. Threonine residuses are crucial for maintaining antifreeze activity. It was found in other AFPs that mutations of the Thrs within these motifs decrease the thermal hysteresis. The Thr hydroxyls define a large flat IBS of 420 Å2, which correlates with high antifreeze activity.
Molecular Basis for Ice Binding
Function
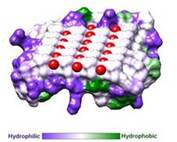
Fig. 2. Hydrophobicity of RiAFP
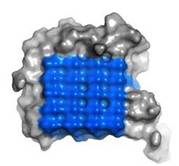
Fig. 3. Ice-binding surface
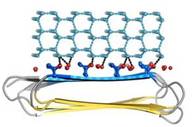
Fig. 4. Docking of RiAFP to the primery prism plane of a hexagonal ice lattice
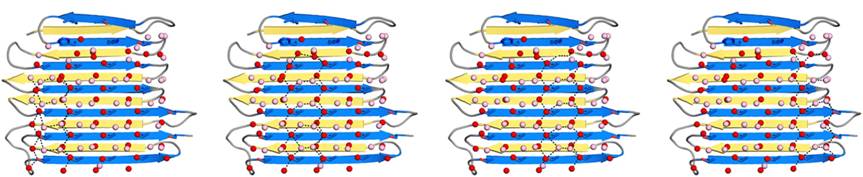
Fig. 5.
Relevance
Structural highlights





