Ubc9 is a ubiquitin conjugating enzyme (E2) whose function involves transfer of ubiquitin or small ubiquitin-like modifier (SUMO) from ubiquitin activating enzyme (E1) to its designated substrate. Ubc9 is specifically involved in SUMO transfer[1].
Structure
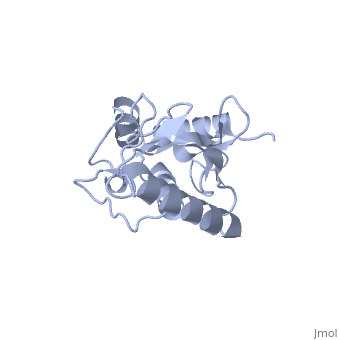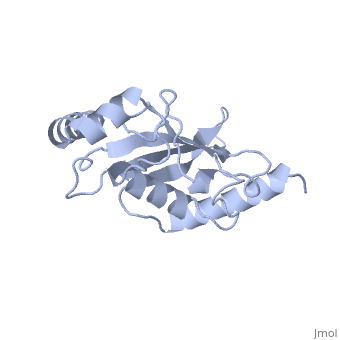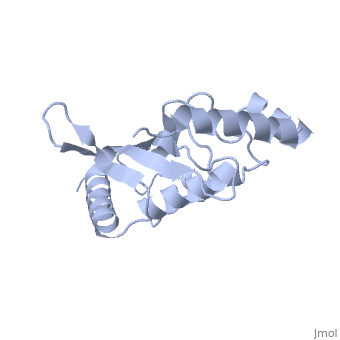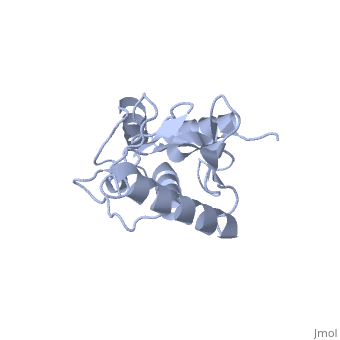
Murine/human Ubc9 exhibits a single domain structure consisting of both alpha helices and beta-pleated sheets (PDB: 1U9A). has been identified as the active residue and is located on a loop of amino acids (78-108) between beta sheet four and alpha helix two [1]. Crystal structure of the (PDB: 2UYZ) have shown that the Ubc9 residues involved in this interaction are located at the end of helix one, on beta strand one, and along the following loop. These residues were shown to interact with the beta sheet of SUMO1 [2].
Function
Ubc9 is a part of the SUMOylation process. It is the enzyme responsible for ligating the SUMO to the protein. Depending on whether the reaction is done in vitro, it will ligate the SUMO directly to the substrate, and if done in vivo, the SUMO will be ligated to the conjugating enzyme and then put onto the substrate [2]. Initially, a thioester bond is formed between the SUMO and the E1 enzyme via an ATP-dependent reaction. The SUMO is then transferred to the active cystein of the E2, in this case, Ubc9. The SUMO is then ligated to a lysine side chain amino group of the substrate, during which an E3 enzyme may or may not be recruited. The use of E3 mediated transfer serves functions such as increasing substrate specificity [2]The Mutagenesis studies on Ubc9 SUMO1 complex have shown that Ubc9H20D and SUMO1E67R mutation cause inhibition of noncovalent interaction between the two, and thus inhibit complex formation. A similar interaction is seen in the Ubc9-SUMO2 complex [2]. Lack of noncovalent interaction between Ubc9 and SUMO2 has been shown to inhibit SUMO2 chain formation. This chain formation is critical for in vivo function, in which formation of ubiquitin or SUMO chains results in cellular processes such as protein degradation [2].
Disease
Expression of Ubc9 has been found to be related to causing human diseases such as cancer, neurodegenerative diseases, and heart diseases. Studies have shown it to interact with tumor suppressor proteins such as p53, p63, and p73 and fundamental in the tumor progression and preventing apoptotic pathways. [3]
Relevance
Structural highlights
Anything in this section will appear adjacent to the 3D structure and will be scrollable.