This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 996
From Proteopedia
Ectatomin (1eci)
Ectatomin (1eci) is the main component of venom of the ant Ectatomma tuberculatum, making up 15%-18% of the crude venom and accounting for 90% of the venom's toxicity.[1] When bitten by E. tuberculatum, Ectatomin inserts into the target's cell membranes and forms a nonselective cation channel.[2] The calculated isoelectric point and molecular weight of Ectatomin are 9.95 and 7928 Da, respectively.[1]
StructureBiologically, Ectatomin exists as a heterodimer stabilized by linkages. The has 37 amino acid residues, while the has 34 amino acid residues. The structure of Ectatomin was solved using 2D NMR and CHARMm computational optimization, though there are 20 similar proposed models in total.[3] Generally, each subunit is composed of two antiparallel α-helices, linked by disulfide bonds, with a connecting hairpin hinge region. The two subunits are linked by a disfulide bond between their hairpin hinge regions. One α-helix from each subunit is kinked approximately 40°, due to the presence of . The kinked α-helix of the α subunit is more kinked, containing three proline residues, while the kinked α-helix of the β subunit only contains one proline residue.[3] The internal region between the two subunits is primarily composed of hydrophobic residues. Sequence - α subunit - GVIPKKIWETVCPTVEPWAKKCSGDIATYIKRECGKL[2] Sequence - β subunit - WSTIVKLTICPTLKSMAKKCEGSIATMIKKKCDK[2] Mechanism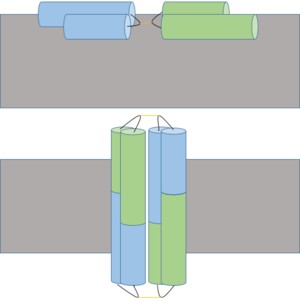 A proposed insertion (above) and dimerization mechanism to form cation channel (below) of Ectatomin in the cell membrane (gray). Insertion occurs when the α and β subunits open at the hairpin hinge region (shown in black with yellow disulfide bonds), exposing internal hydrophobic residues which interact with hydrophobic lipid tails of the cell membrane. Pore formation occurs after dimerization, allowing ions to freely cross the membrane. Ectatomin has several proposed mechanisms of action. The primary proposed mechanism involves the formation of a nonselective cation channel. In this mechanism, the α and β subunits open up, exposing the internal hydrophobic residues.[3] The protein flattens while remaining attached at the hairpin hinge region. The now exposed hydrophobic residues nonselectively insert into plasma membranes.[3] The inserted protein dimerizes, eventually forming a nonselective cation channel.[1] For the second and third proposed mechanisms of action, Ectatomin has also been shown to inhibit kinases, specifically protein tyrosine kinase and protein kinase C, and natural Ca2+ channels. Kinase inhibition would potentially allow Ectatomin to interfere with various components of signal transduction.[1] Calcium channel inhibition would potentially allow Ectatomin to affect physiological processes such as contraction, neurotransmitter release and neuronal activity regulation.[4] ToxicologyWith an LD50 of 6.8 μg kg-1, Ectatomin is one of the deadliest proteins known to man, alongside Tetanospasmin and the Botulinum toxin.[1] Ectatomin attacks and forms pores in the plasma membrane, allowing cations to freely move in and out of the cell.[3] The primary cations which flow through the pore are Ca2+ and K+. Physiologically, the primary targets of Ectatomin are muscle cells, particularly the heart, and neurons. As the cation concentration equilibrates between the two sides of the cell membrane, the affected cells lose their ability to maintain and form a membrane potential and concentration gradient.[2] Without the ability to form a calcium or potassium gradient, muscle cells are not able to contract and the target is rendered paralyzed. This is particularly dangerous when the heart is affected as it halts the circulatory system.[2] When neurons are affected, the inability to maintain and form a membrane potential eliminates the ability of the neuron to receive and transmit information.[1] References
| ||||||||||||
