S-crizotinib ligand</scene>. Crizotinib is a tyrosine kinase inhibitor, and its molecular structure is C21H22Cl2FN5O. The stereochemistry of the crizotinib determines if this inhibitor prevents MTH1 from carrying out its purpose in cancer cells; the S-crizotinib stops MTH1 from working while the R-crizotinib has no effect on the protein (3,7).
Binding Interactions
Human MutT homologue, also known as MTH1, is a nucleotide pool sanitization enzyme and the target for crizotinib, a competitive inhibitor. Crizotinib is a kinase inhibitor that suppresses MTH1 activity in concentrations as low as nanomoles/liter, suggesting high levels of specificity (1). Crizotinib acts on MTH1 through competitive inhibition, meaning it displaces kinases, the usual substrates. Because the specificity of the binding pocket of MTH1 depends on the stabilization of the enol tautomer of 8-oxo-dGTP, crizotinib’s chemistry interacts with this conformation to be effective (2). The binding of crizotinib yields no major structural changes in the MTH1 protein.
The most significant interactions between crizotinib and the MTH1 active site are hydrogen bonding interactions. Hydrogen bonding occurs between the Asparagine residue 33 and the N3 of 8-oxo-dGMP and between the acidic Aspartate residues 119 and 120 and the oxygen in 8-oxo-dGMP. Both the Asp 119 residue and the Asn 33 residue are crucial to binding specificity (2). Because crizotinib has a chiral center, the (S) and (R) enantiomers act differently in the binding pocket of MTH1. After recent testing of MTH1 using both enantiomers, the (S) enantiomer has shown a higher affinity for the MTH1 binding pocket, whereas the (R) enantiomer is a better inhibitor for most other similar protein kinases. An eclipsed conformation of a methyl group at the chiral center and a chlorine attached to the benzyl ring are responsible for reducing the energetic favorability of the (R) enantiomer in the binding pocket of MTH1 (1).
Additional Features
The can be seen inside the globular protein structure. The majority of the residues appear exposed on the protein surface rather than buried within the protein. Acidic and basic residues are also prevalent within the binding pocket.
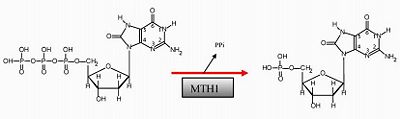
MTH1 structurally recognizes and distinguishes the 8-oxo-dGTP from 8-oxo-dGMP different from one another. In order to understand how MTH1 functions, this is essential to explore. The protein MTH1 has been co-crystallized with the substrate 8-oxo-dGTP and the structure of the crystal was observed (2). A reaction took place and the product, 8-oxo-dGMP, was bound in a pocket formed by the . This green scene representation is not exact, and a structural change may have occurred upon 8-oxo-dGTP binding. make hydrogen bonds to the 2-amino group of 8-oxo-dGMP. This amino position is central for the interaction. Nucleotide analogs were used to test MTH1 substrate recognition; results were in agreement. Removal of the 2-amino group led to a 10-fold less effective degradation (2). Mg2+ also appeared in the co-crystal structure, but the electron density, coordination geometry, and coordination distances showed Mg2+ was not bound to the complex. It was hypothesized that Mg2+ may coordinate with the substrate of the triphosphate nucleotide (2). Many other residues involved in specificity and recognition have been identified.
MutT is a homolog of MTH1, shares the same nubix family, and is expressed in Escherichia coli. MutT also catalyzes the hydrolysis of 8-oxo-dGTP to 8-oxo-dGMP, but has a 21% (low) sequence identity to MTH1. Co-crystal structure produced the anti conformation of bound 8-oxo-dGMP in MTH1 while syn conformation was observed in MutT (2). From the crystal structure it was determined that the base–protein interactions are quite different and reveal no conservation of structure. Although the proteins shared a conserved catalytic region, the nucleotide recognition is not conserved. Diverging evolutionary routes have been taken, and one possibility is to allow MTH1 to evolve more non-specific substrate specificity (2). Understanding the mechanisms of non-specific substrate binding could be an important step to developing new inhibitors for similar enzymes.
Quiz Question 1
- This scene colors the protein from the N-terminus to the C terminus.
- This scene shows the active site of the protein. Individual amino acids are labelled and active site residues are colored according to charge (red = positive, blue = negative, white = uncharged). The ligand (S-crizotinib) in the center is colored by atom (Black = Carbon, Red = Oxygen, Blue = Nitrogen, Green = Halogen).
Using the green scenes provided above, answer the following:
a.) Suppose there is a point mutation causing a change at ASP-120 in MTH1. Which of the following amino acids substitutions would be least likely to affect substrate binding? Explain your reasoning.
1.) Lysine (LYS)
2.) Glutamate (GLU)
3.) Glycine (GLY)
b.) If there were a point mutation at PHE-27, which of the following amino acid substitutions would likely affect ligand binding the most? Again, explain your reasoning.
1.) Tryptophan (TRP)
2.) Tyrosine (TYR)
3.) Arginine (ARG)
Quiz Question 2
The above two green scenes highlight the chiral center of the MTH1 inhibitor called crizotinib. Research has shown that the s-enantiomer binds with more affinity to it's MTH1 substrate but that it is not due to interaction with the protein itself at the binding site.
Can you see an unfavorable interaction near the chiral center in the r-enantiomer that does not exist in the s-enantiomer? Explain.
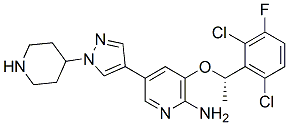
Crizotinib Structure Modified from adooq.com
See Also
- MTH1 (7,8-dihydro-8-oxoguanine triphosphatase)
Credits
- Introduction - Megi Marina
- Overall Structure - Cassandra Martin
- Drug Binding Site - Ben Ryter
- Additional Features - Eric Rice
- Quiz Question 1 - Matt Tuttle
- Quiz Question 2 - Colin Hannahan
References
- Huber, Kilian V. M., Eidarus Salah, Branka Radic, Manuela Gridling, Jonathan M. Elkins, Alexey Stukalov, Ann-Sofie Jemth, Camilla Göktürk, Kumar Sanjiv, Kia Strömberg, Therese Pham, Ulrika Warpman Berglund, Jacques Colinge, Keiryn L. Bennett, Joanna I. Loizou, Thomas Helleday, Stefan Knapp, and Giulio Superti-Furga. "Stereospecific Targeting of MTH1 by (S)-crizotinib as an Anticancer Strategy." Nature 508 (2014): 222-27. Web.
- Svensson, Linda M., Ann-Sofie Jemth, Mathhieu Desroses, Olga Loseva, Thomas Helleday, Martin Higbom, and Pal Stenmark. "Crystal Structure of Human MTH1 and the 8-oxo-dGMP Product Complex." Http://www.sciencedirect.com. N.p., 19 Aug. 2011. Web.
- "Crizotinib | C21H22Cl2FN5O - PubChem." Crizotinib | C21H22Cl2FN5O - PubChem. N.p., n.d. Web. 03 Apr. 2015.
- Fricker, Janet. "Two Studies Show MTH1 Offers Promising New Target for Cancer Treatment." . Ecancer. Cancer Intelligence, 02 Apr. 2014. Web. 03 Apr. 2015.
- M. Mishima,; Y. Sakai,; N. Itoh,; H. Kamiya,; M. Furuichi,; M. Takahashi, Y. Yamagata, S. Iwai,; Y. Nakabeppu,; and M. Shirakawa. “Structure of the Human MTH1, a Nudix Family Hydrolase that Selectively Degrades Oxidized Purine Nucleoside Triphosphates”, J Biol Chem., 279, no. 32, 33806-33815, 2004.
- S.B. Gabelli,; M.A. Bianchet,; M.J. Bessman,; L.M. Amzel “The structure of ADP-ribose pyrophosphates reveals the structural basis for the versatility of the Nudix family”, Nat Struct Biol, 8, 467-472, 2001.
- "Crizotinib, (S)-." (S)-Crizotinib. N.p., n.d. Web. 03 Apr. 2015.