Introduction
FAS-II System
Mechanism of Action
Structure
Crystal structures of InhA reveal a (each subunit featured with a different color) with separate ligand binding sites in each subunit. Each subunit features a single domain with a central core that supports a NADH binding site. The alpha6 and alpha7 helices of the monomer form one side of the fatty acyl substrate binding crevice of InhA. Within this binding crevice, many hydrophobic side chains from the amino acids involved in forming the substrate binding loop, , interact with the substrates.
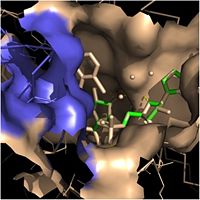
Fatty Acyl Binding Crevice
One side of this cavity is open and exposed to solvent, which allows the substrates to access the binding pocket of this enzyme. The size of the substrate binding loop is a primary determinant of the ability of InhA to distinguish between shorter and longer substrates and provide for the enoyl-ACP reductase reaction.
Fatty Acyl Binding Crevice
In the substrate binding pocket, NADH sits on the top shelf of the fold, and the fatty acyl substrate sits on top of NADH. Both molecules are held in place by interactions with amino acid residues in the substrate binding loop.
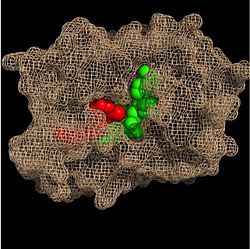
Substrate Binding Pocket (NADH in green; fatty acyl substrate in red)
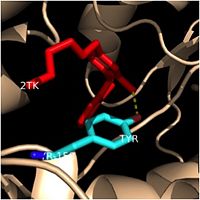
Catalytic Triad
Hydrogen Bonding Interactions
.
Clinical Applications
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.