General Structure
Antigen 85C was crystallized in its dimeric form.[3] The shown in the monomeric form is composed of helices with one interwoven beta sheet. Due to the serine hydrolase activity of Ag85C, the enzyme contains a ‡/? hydrolase fold with a central ?-sheet bordered by ‡?helices, and this tertiary conformation is highly conserved across enzymes that function in this capacity. [4] The substrate binding pocket of Ag85C is composed of two separate but equally important components; there is carbohydrate binding pocket for the trehalose, and there is a fatty acid binding pocket for the mycolic acid. As a result, trahalose monomycolate can effectively bind to the Ag85C binding pocket.
Enzymatic Activity
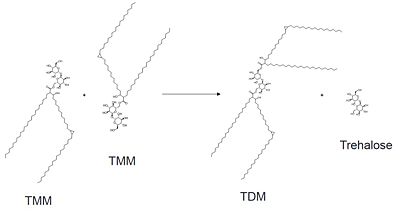
Mutagenesis studies have confirmed the Ag85C functions through a Glu-His-Ser , similar to that of chymotrypsin. By modifying each of the catalytic residues separately testing the enzyme?s relative activity, it has been shown that mutation of any one of these residues dramatically reduces activity (Figure #). The S124 alcohol?s nucleophilicity is inductively strengthened through H260 and E224, which allows S124 to hydrolyze trehalose 6, 6?-dimycolate. The formation of the functional catalytic triad relies on upon Van der Waals interaction between C209 and the peptide bond between L232 and T231. This interaction results in a kinked conformation of the ‡9 helix, which promotes that activity of the catalytic triad. As a result, Ag85C, a mycolyl transferase, can facilitate the modification of trehalose monomycolates to trehalose dimycolates, which are then transported to the bacterial cell wall.
Methods of Inhibition
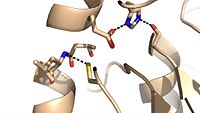
Cys209 stabilizing kinked formation of alpha-9 helix
Due to the importance of Ag85C enzymatic activity in maintaining the integrity of the mycobacteria tuberculosis cell wall though mycolic acid modifications, the Ag85C enzyme represents a potentially effective avenue for inhibiting cell growth. The conformational sensitivity of the active site residues, H260, E228, and S124, relies entirely upon Van der Waals interaction between C209 and L232-T 231 (Figure #). The C209 facilitated interaction causes the to acquire a kinked conformation that promotes optimal interaction distances between catalytic residues. As a result, C209 has been a specific target residue for Ag85C inhibition.>
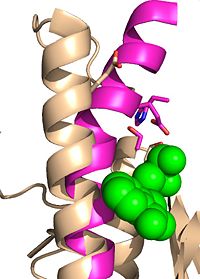
Ebselen inhibition relaxing the alpha-9 helix
Ag85C can be inhibited by ebselen covalently bound to the sulfur of C209. Ebselen is a thiol-modifying agent that serves as an electrophile for the C209 that results in a sulfur-selenium bond. Co-crystallization of ebselen with Ag85C provides an explanation for the mechanism of ebselen-based inhibition. The addition of ebselen increases the distance between C209 and L232-T31, which effectively disrupts the interaction that holds the ‡9 helix in the active conformation. Furthermore, the bulk of ebselen creates steric hindrance with the ‡9 helix residues (Figure #). Relaxation of the ‡9 helix removes E228 and H260, which now interacts with S148, from the active site. The absence of these residues decreases the nucleophilicity of the S124 alcohol which decreases serine hydrolytic activity.
Inhibitors
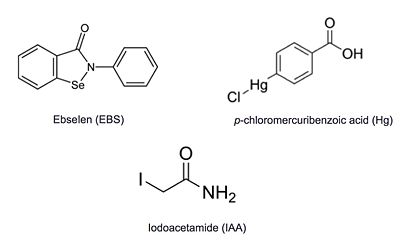
Additional thiol-modifying agents, p-chloromercuribenzoic acid and iodoacetamide, were crystalized with Ag85C. The structures show that each of these thiol-reactive inhibitors covalently bound to C209 and caused a relaxation of the ‡9 helix in a similar fashion to ebselen.