General Structure
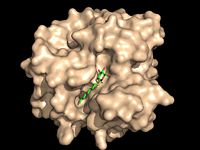
Figure 1: Octylthioglucoside, a substrate analog, shown in the binding pocket of
Ag85C Antigen 85C was crystallized in its dimeric form.[3] The shown in the monomeric form is composed of helices, shown in pink, with one interwoven beta sheet, shown in yellow. The confrontation of a central β-sheet bordered by α–helices creates an a α/β hydrolase fold in Ag85C, and this tertiary conformation is highly conserved across enzymes that function in this capacity. [4] The substrate binding pocket of Ag85C is composed of two separate but equally important components; there is carbohydrate binding pocket for the trehalose, and there is a fatty acid binding pocket for the mycolic acid. As a result, trehalose monomycolate can effectively bind to the Ag85C binding pocket. This binding pocket is shown in the left image with a substrate mimic.
Enzymatic Activity
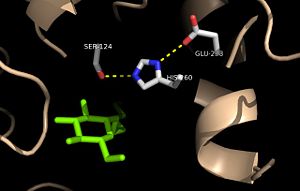
Figure 2: Relation of the catalytic triad to the octylthioglucoside analog in
Ag85C Mutagenesis studies have confirmed the Ag85C functions through a Glu-His-Ser , similar to that of chymotrypsin. By modifying each of the catalytic residues separately testing the enzyme’s relative activity, it has been shown that mutation of any one of these residues dramatically reduces activity. The S124 alcohol’s nucleophilicity is inductively strengthened through H260 and E224, which allows the S124 residue to catalyze a reaction that involves trehalose 6, 6’-dimycolate. The formation of the functional catalytic triad relies on upon Van der Waals interaction between C209 and the peptide bond between L232 and T231. This interaction results in a kinked conformation of the α9 helix, which promotes that activity of the catalytic triad. As a result, Ag85C, a mycolyl transferase, can facilitate the modification of trehalose monomycolates to trehalose dimycolates, which are then transported to the bacterial cell wall. This reaction is shown in Figure 3 below.

Figure 3: General reaction catalyzed by Antigen 85C
Methods of Inhibition
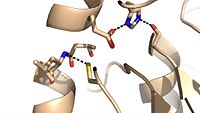
Figure 4: Cys209 stabilizing kinked formation of alpha-9 helix in the native
Ag85C enzyme
Due to the importance of Ag85C enzymatic activity in maintaining the integrity of the Mycobacteria tuberculosis cell wall though mycolic acid modifications, the Ag85C enzyme represents a potentially effective avenue for inhibiting cell growth. The conformational sensitivity of the active site residues, H260, E228, and S124, relies entirely upon Van der Waals interaction between , which can be seen in both the scene and Figure 4. The C209 facilitated interaction causes the to acquire a kinked conformation that promotes optimal interaction distances between catalytic residues. As a result, C209 has been a specific target residue for Ag85C inhibition.
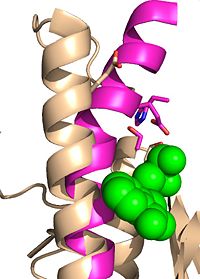
Figure 5: Ebselen inhibition relaxing the alpha-9 helix in
Ag85C-Ebselen
Ag85C can be inhibited by ebselen, which covalently bounds to the sulfur in C209. Ebselen is a thiol-modifying agent that serves as an electrophile for a C209 nucleophilic attack that results in sulfur-selenium bond formation. Co-crystallization of ebselen with Ag85C provides an explanation for the mechanism of ebselen-based inhibition. The addition of ebselen increases the distance between C209 and L232-T231, which effectively disrupts the interaction that holds the α9 helix in the active conformation. The disruption of this interaction causes the α9 helix to . Furthermore, the bulk of ebselen creates steric hindrance with the α9 helix residues, which can be seen in Figure 5. The pink helix represents the native enzyme, and the tan helix represents Ag85C covalently bound to ebselen, which is shown in green. Relaxation of the α9 helix due to ebselen removes E228 and H260, which now interacts with S148, from the active site. The absence of these residues destroys the charge relay mechanism, and as a result, the nucleophilicity of the S124 alcohol is not longer strengthened, which decreases serine hydrolytic activity.
Inhibitors
Additional thiol-modifying agents, p-chloromercuribenzoic acid and iodoacetamide, were crystalized with Ag85C. The structures show that each of these thiol-reactive inhibitors covalently bound to C209 and caused a relaxation of the α9 helix in a similar fashion to ebselen.
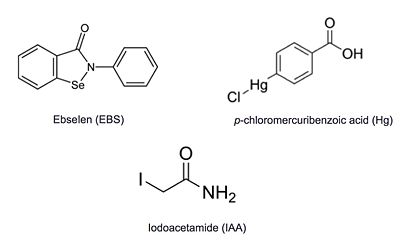
Figure 6: Known thiol-reactive inhibitors of Ag85C






