Biological Role
Cell Wall of Mycobacteria
The unusually thick and waxy cell wall of mycobacteria is primarily composed of peptidoglycans, arbinogalactans, and mycolic acids. The mycolic acids, which have very long carbon chains and exhibit extreme hydrophobicity, are responsible for forming the outermost layer of the cell wall, thus creating a hydrophobic envelope surrounding the mycobacterium. Much of the mycolic acid content of the cell wall is in the form of esters (trehalose-6-monomycolate, TMM) and bis-esters (trehalose-6,6'-dimycolate, TDM, cord factor) of trehalose.
Enzymatic Activity
The antigen 85 (Ag85) complex in Mycobacterium tuberculosis is composed of three homologous proteins: Ag85A, Ag85B, and Ag85C, encoded by the fbpA, fbpB, and fbpC genes, respectively. Each of these Ag85 components (A, B, and C) is an acyltransferase enzyme (EC 2.3.1.122) that can catalyze mycolyl transfer from TMM to another alcoholic substrate, including TMM itself (Figure 1).
Figure 1: A mycolyl transfer reaction catalyzed by Ag85C
Clinical Relevance
The hydrophobic envelope created by these inclusion of mycolic acids in the cell walls of mycobacteria results in a barrier against small hydrophilic molecules, such as Tuberculosis antibiotics. Thus, inhibition of Ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant Mycobacteria tuberculosis. [1]
Structure of Wild Type Enzyme
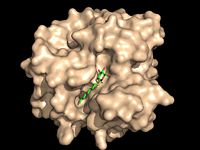
Figure 2A: Octylthioglucoside (green), a substrate analog, shown in the binding pocket of Ag85C (tan, from
1va5)
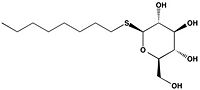
Figure 2B: 1-
O-Octyl-β-D-thioglucoside
Wild type Ag85C, in the absence of any specific ligands, was originally crystallized as a .[2] The (shown in the monomeric form) is 38% α-helical (pink) and 16% β-sheet (yellow). The confrontation of a central β-sheet bordered by α–helices comprises an α/β hydrolase fold in Ag85C, a supersecondary structure that is highly conserved across serine hydrolases. The active site of Ag85C is composed of two adjoined binding pockets; there is carbohydrate binding pocket for the trehalose substrate, and there is a fatty acid binding pocket for the mycolic acid. As a result, trehalose monomycolate can effectively bind to the Ag85C active site. This active site is shown in Figure 2, in which Ag85C is shown with a bound substrate mimic, octylthioglucoside[3] (Figure 3).
Catalytic Triad
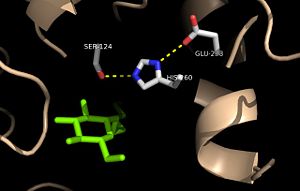
Figure 3: Relation of the catalytic triad to the octylthioglucoside analog in
Ag85C Mutagenesis studies[4] have confirmed the the catalytic function of Ag85C is dependent upon a Glu-His-Ser , similar to that of chymotrypsin. By modifying each of the catalytic residues separately and then testing the variant enzyme’s relative activity, it has been shown that mutation of any one of these residues dramatically reduces activity. The S124 alcohol’s nucleophilicity is inductively strengthened via deprotonation by H260 and E224, which allows the S124 residue to catalyze the reaction (Figure 4).
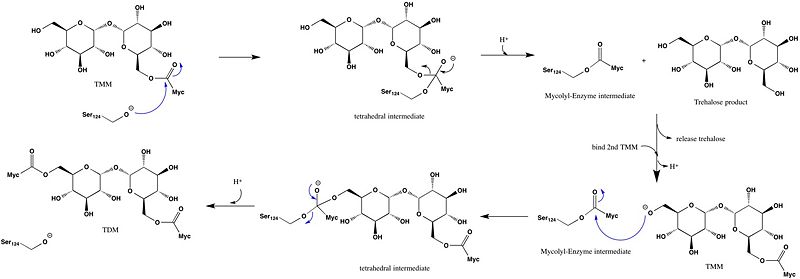
Figure 4: Catalytic mechanism
Cys 209
is located near the Ag85C active site, but is not directly involved in the catalytic mechanism. However, formation of a functional catalytic triad relies on upon a . The C209 facilitated interaction stabilizes a that promotes optimal interaction distances between catalytic residues.
Structures of Variant Enzymes
Covalent Modification of Cys209
Ag85C has been crystallized following covalent inhibition by several thiol-modifying agents: ebselen, p-chloromercuribenzoic acid, and iodoacetamide. Each of these thiol-reactive inhibitors covalently bound to C209 and caused a relaxation of the α9 helix. The resulting relaxed conformation of the helix significantly reduces or completely eliminates the enzymatic function of Ag85C.[5]
Modification by Ebselen
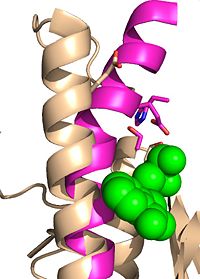
Figure 6: Ebselen inhibition relaxing the alpha-9 helix in
Ag85C-Ebselen Ag85C can be inhibited by ebselen, which covalently bounds to the sulfur in C209. Ebselen is a thiol-modifying agent that serves as an electrophile for a C209 nucleophilic attack that results in sulfur-selenium bond formation. Co-crystallization of ebselen with Ag85C provides an explanation for the mechanism of ebselen-based inhibition. The addition of ebselen increases the distance between C209 and L232-T231, which effectively disrupts the interaction that holds the α9 helix in the active conformation. The disruption of this interaction causes the α9 helix to . Furthermore, the bulk of ebselen creates steric hindrance with the α9 helix residues, which can be seen in Figure 5. The pink helix represents the native enzyme, and the tan helix represents Ag85C covalently bound to ebselen, which is shown in green. Relaxation of the α9 helix due to ebselen removes E228 and H260, which now interacts with S148, from the active site. The absence of these residues destroys the charge relay mechanism, and as a result, the nucleophilicity of the S124 alcohol is not longer strengthened, which decreases serine hydrolytic activity.
Mutation of Cys209
E228Q
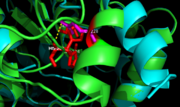
Figure 7. Ag85C-E228Q active site. The glutamate residue is shifted four angstroms in the mutated form of this enzyme causing a rearrangement of hydrogen bonds within the enzyme. The histidine residue, labeled in pink, takes on two conformations, binding with alternative serine residues, labeled in red.
The mutation introduced in Ag85C-E228Qcauses the Glu228 of the catalytic triad to be shifted 4 angstroms from its original position in the native structure of Ag85C (Figure 7). Due to the shift of Glu228 is the loss of hydrogen bonds between Ser124 and His260. Instead, , which also results from the shift of Glu228. A weak electron density difference in the His260 position of the native and mutated structures was also noted, suggesting that the residue may take on two alternative conformations in the mutant. Overall, the enzyme functionality is decreased to only 17% activity.[5]
Unlike other Ag85C mutants and modifications aforementioned, the does not eliminate any hydrogen bonds in the catalytic triad. Rather, it simply replaces a carboxylate moiety with an amide. Further, the structural change observed in the low-energy conformation of the mutant provides additional support for the hypothesis that the natively kinked helix α-9 is central to the enzymatic function of Ag85C.[5]
H260Q
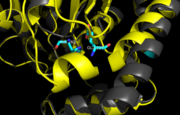
Figure 8. Ag85C-H260Q active site. A shift in helix α-9 prevents formation of any stabilizing hydrogen bonds between residues His260 and Glu228, thus decreasing its enzymatic activity.
In , a shift in helix α-9 between residues His260 and Glu228, thus decreasing its enzymatic activity (Figure 8). The conversion of the glutamate, a key player in the catalytic triad, to the corresponding amide-containing side chain as well as a loss of a general base in the charge relay are both key causes for the loss of function.[5]
Additional thiol-modifying agents, p-chloromercuribenzoic acid and iodoacetamide, were crystalized with Ag85C. The structures show that each of these thiol-reactive inhibitors covalently bound to C209 and caused a relaxation of the α9 helix in a similar fashion to ebselen.
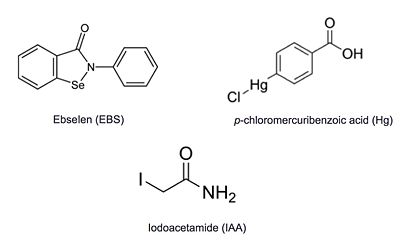
Figure 6: Known thiol-reactive inhibitors of Ag85C









