User:Michael Adams/Sandbox 1
From Proteopedia
|
This is a default text for your page Michael Adams/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs. You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Contents |
Isolation Methods
In Strong and Ellington’s 1994 experiment, arginine kinase (AK) was isolated from Limulus polyphemus, the Atlantic horseshoe crab, a marine chelicerate arthropod. They isolated the gene for AK and sequenced the DNA and produced a full genome breakdown of the 1071 nucleotide gene. The 1071 nucleotides translate to a 357 amino acid protein that is extensively similar to AK’s extracted from other organisms. It also provides a similar function to that of creatine kinase, in vertebrates (Strong and Ellington, 1994).
Structural highlights
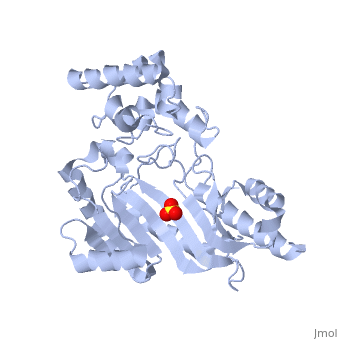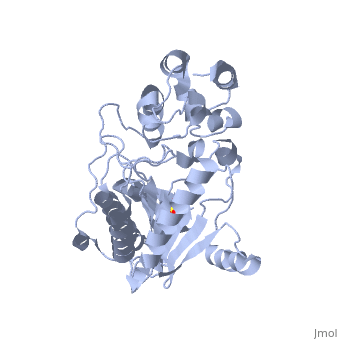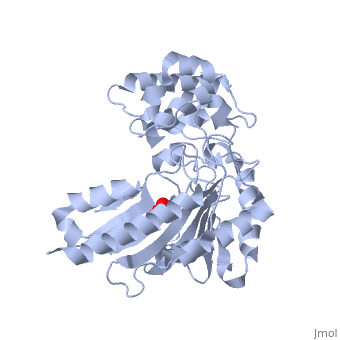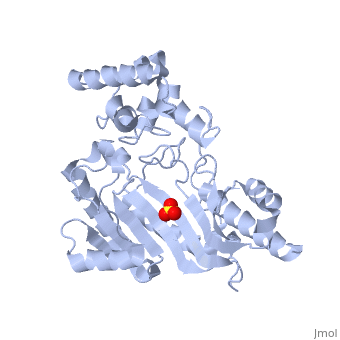
The structure of arginine kinase is mainly α-helical and contains an N-terminal region (Figure 1b). However, when compared to creatine kinase, arginine kinase is not terminated with a pair of proline-glycine. Proline typically restricts change in conformation and is the amino acid that terminates helices, while glycine is associated with flexibility. On the C-terminal end, there are eight-stranded antiparallel β-sheets with seven α-helices flanking them (Figure 1). The small domain specificity loop forms a “specificity” pocket surrounding the methyl substituent of the guanidinium group that is unique to creatine substrates. In this region, five residues differ between arginine and creatine kinases: 312, 314, 315, 317, and 319 (Newsholme et al, 1978). Within each arginine kinase, there is typically a Mg+2 ion adjacent to the antiparallel β-sheet (Figure 1b). Typically two arginine kinase structures mirror each other and form a hole like structure in between the two. However, when a substrate is in the binding site, the active site remains unchanged and does not change in conformation (Figure 1a).
Function
Arginine Kinase is part of a class of kinases that regulates ATP levels in the body to help maintain homeostasis. It creates a sort of storage option for ATP. It is the most common phosphokinase (PK) in invertebrates. The most common PK in vertebrates is Creatine Kinase (Pereira et al, 2000). Arginine Kinase is a phosphokinase - a kinase used in the catalyzation of phosphagens and adenosine diphosphate (ADP) into adenosine triphosphate (ATP). Phosphagens act as a storage form of phosphate (Nω-phospho-L-arginine) that can be catalyzed into an energy source (ATP) when needed (Pereira et al, 2000). The arginine kinase is a lock and key catalyst that holds ADP and phosphoarginine in place and catalyzes the transfer of inorganic phosphate on phosphoarginine to ADP and forms of arginine and ATP (Azzi et al., 2004).
Application to the Animal Kingdom
Arginine Kinase is the individual phosphagen kinase that is found in major invertebrates, such as: arthropods, molluscs, and echinoderms. Most recently, an arginine kinase was purified from a house fly and a crystalline preparation was obtained from the thorax of a honey bee. This gave Wallimann and Eppenberger the initiative to investigate the arginine kinase in Drosophila melanogaster. Since the genome and genetic development of Drosophila melanogaster is well understood, this allows for any discoveries made to be easily interpreted. Additionally, further discoveries will help better understand the characteristics of arginine kinase corresponding vertebrate enzyme, creatine kinase (Wallimann et al., 1973) Arginine Kinase (AK) is represented by a single gene and the sequence or partial sequence is available from Drosophila, Limulus, lobster, shrimp, and abalone (Wang et al., 1998). In the study by Wang et al. 1998, a phylogenetic tree was put together for the evolution of Arginine Kinase compared to Creatine Kinase. It indicates that there are major clusters corresponding to Creatine Kinase and Arginine Kinase. In the Arginine Kinase cluster, grasshopper is more similar to Drosophila than to lobster or horseshoe crab. Also, it is expressed in the gills of two species of euryhaline crabs, the blue crab Callinectes sapidus and the shore crab Carcinus maenus, in which energy-requiring functions include monovalent ion transport, acid-base balance, nitrogen excretion (Kotylar et al. 2000). Arginine Kinase is the major phosphagen kinase found in invertebrates.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
Azzi, A., Clark, S. A., Ellington, W. R., & Chapman, M. S. (2004). The role of phosphagen specificity loops in arginine kinase. Protein Science : A Publication of the Protein Society,13(3), 575–585. http://doi.org/10.1110/ps.03428304
Kotlyar, S., Weihrauch, D., Paulsen, R., & Towle, D. (2000, July 20). Expression of Arginine Kinase Enzymatic Activity and mRNA in Gills of the Euryhaline Crabs Carcinus Maenas and Callinectes Sapidus. Retrieved November 17, 2015, from http://jeb.biologists.org/content/jexbio/203/16/2395.full.pdf
Newsholme, E. A., Beis, I., Leech, A. R., & Zammit, V. A. (1978). The role of creatine kinase and arginine kinase in muscle. Biochemical Journal, 172(3), 533–537.
Pereira, C. A., Alonso, G. D., Paveto, M. C., Iribarren, A., Cabanas, M. L., Torres, H. N. & Flawia, M. M. (2000) Trypanosoma cruzi arginine kinase characterization and cloning., J. Biol.Chem. 275, 1495-1501.
Strong, S., & Ellington, W. (1994). Isolation and sequence analysis of the gene for arginine kinase from the chelicerate arthropod, Limulus polyphemus: Insights into catalytically important residues. Biochimica Et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology, 197-200.
Wallimann, Theo, and Hans M. Eppenberger. "Properties of Arginine Kinase from Drosophila Melanogaster." Www.onlinelibrary.wiley.com. Laboratory of Developmental Biology, Swiss Federal Institute of Technology, Zurich, 21 June 1973. Web. 12 Nov. 2015
Wang, Yu-mei E., Pia Esbensen, and David Bentley. "Arginine Kinase Expression and Localization in Growth Cone Migration." The Journal of Neuroscience 18.3 (1998): 987-98. Web. 15 Nov. 2015. <http://www.jneurosci.org/content/18/3/987.full.pdf>.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644