User:Elisha Lacey/Sandbox 1
From Proteopedia
The human genome has 56 bromodomains across 42 proteins. Bromodomains are found in mammals.
Contents |
Bromodomain testis specific
| |||||||||||
</StructureSection>
Function
Bromodomain testis specific is a monomer that is only fully functional as a dimer. BRDT is a transcriptional regulator,located in the nucleus, for gene expression during spermatogenesis. BRDT is important to healthy progeny, without it sperm is unable to correctly compact genome. BRDT gives rise to the shape of sperm cells, without it the sperm cells are misshapen.
BRDT and fertility
Loss of one of the bromodomains or even single point mutations leads to decrease spermatogenesis and defective sperm. If both bromodomains are knocked out it leads to loss of fertility.
Relevance
Male contraception has been eluding scientists for years and is a desired area to prevent unplanned pregnancies. In studies of an anti-cancer drug triazolotheinodiaepine known as JQ1 that inhibits BRD4 in cancer pathogenesis. In experiments in mice it is seen, due to structural similarities, JQ1 blocks the production of sperm in testes. The binding of JQ1 in the acetyl-lysine pocket prevents recognition of acetylated histone H4KAC4. JQ1 in studies is not seen to affect hormone levels(leutinizing hormone. follicle stimulating hormone, testosterone), mating behaviors or harmful effects to eventual offspring. Also JQ1 is not seen to cause sterility in mice. The effects of JQ1 on fertility are reversible and dose and time dependent effects on fertility. In mice it reduces size of testis, seminiferous tubes, and number of spermatozoa as well as the mobility of spermatozoa.
This is a chemical representation of the JQ1 molecule.
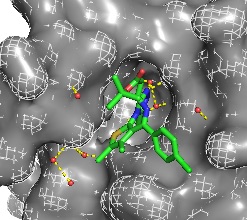
This image is a surface representation of BRDT with JQ1 in the binding pocket to see how JQ1 fits into the binding pocket.
Analysis of Structure
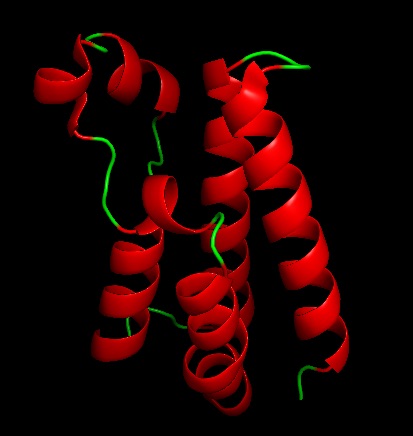
The functional unit for BRDT is two bromodomain motifs, each monomer is composed of 4 anti-parallel alpha helixes. The 2 monomers are not unique to one another. At the end of the helix bundles is the acetylated-lysine binding site binding site. BRDT belongs to the histone acetyltransferase superfamily. BRDT is tissue restricted to the testis. BRDT activates the histones by hyper acetylation at the promoter of genes leading to condensed acetylated chromatin of a haploid spermatid. Running an alignment gives 200 sequence similarities which shows that this sequence is highly conserved among organism in the phylogenic tree. This was also seen when running multiple alignments and the sequence was similar among several different organisms.
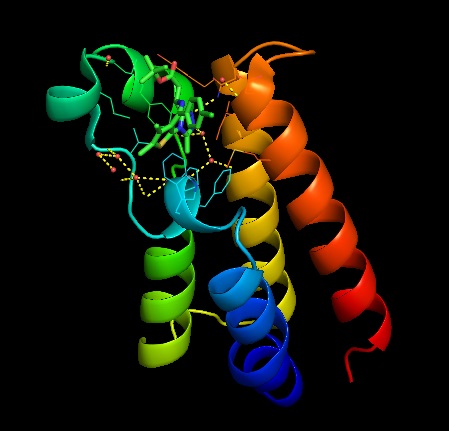
This image is a the BRDT monomer with ligand bound to visual the binding pocket present in the monomers.
Analysis of related Sequences
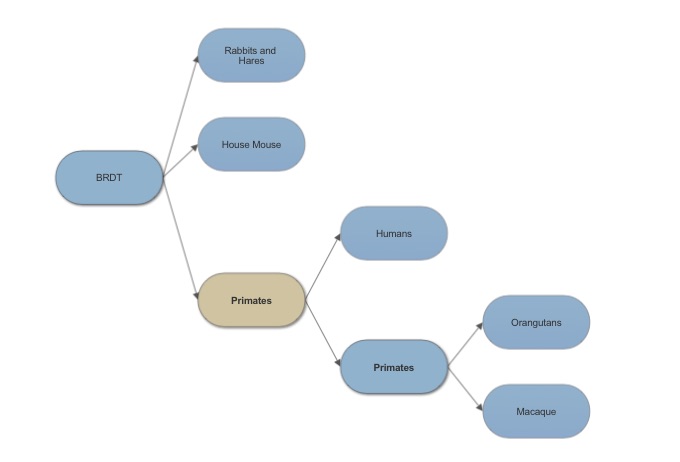
Bromodomain testis specific in humans shares 97% sequence identity with bromodomain testis specific in Sumatran Orangutans. It also shows 99% sequence similarity. These genes are close orthologs with such similar sequence identity and they both serve a function in fertility. The most distant sequence alignment is a black flying fox with and identity of 89% and still is an ortholog to BRDT in humans. Looking at an alignment comparison of BRDT and 16 other proteins the representation shows a sequence match of the alpha helixes and the non aligned segments as being random coils connecting the helices.
This image above is just a short segment of aligned sequence to illustrate the sequence similar regions, going down the rows are different sources of DNA in the phylogenic tree. In red are the aligning codes and in grey are the non match sequences. The image was included to illustrate a part of the conserved sequence among orthologs.
Evolution of BRDT
Looking at the evolution of BRDT it is easy to see why mice are a choice of animal model for assessing fertility affects due to BRDT.
References
PDB ID:4FLP
http://dx.doi.org/10.2210/pdb4flp/pdb
Berkovits B, Wolgemuth D. The first bromodomain of the testis-specific double bromodomain protein Brdt is required for chromocenter organization that is modulated by genetic background. PMC. 2011 Dec 15: 360 (2):358-368.
Barda S, Yogev L, Paz G, et al. BRDT gene sequence in human testicular pathologies and the implication of its single nucleotide polymorphism (rs3088232) on fertility. Andrology. 2014 May 28; 2(4): 641-647.
H.M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N. Shindyalov, P.E. Bourne (2000) The Protein Data Bank Nucleic Acids Research, 28: 235-242.
Madden T. The BLAST Sequence Analysis Tool. 2002 Oct 9 [Updated 2003 Aug 13]. In: McEntyre J, Ostell J, editors. The NCBI Handbook [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2002-. Chapter 16. Available from: http://www.ncbi.nlm.nih.gov/books/NBK21097/
Zdrojewicz Z, Konieczyn R, Papier P, Szten F.Brdt Bromodomains Inhibitors and Other Modern Means of Male Contraception. ACEM. 2015 Feb 23; 24(4):705-714.