Cholera toxin (CTX), secreted by bacterium Vibrio Cholerae,is an oligomeric complex of enzymatic A and pentameric B subunits, which bind to the cell surface. CTX is the main cause of the diarrhea symptoms of cholera infection.
Structure
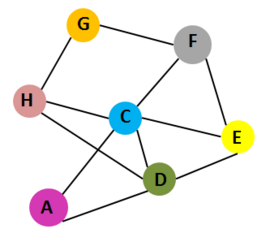
Interaction of 7 chains of Cholera toxin
1xtc. Cholera toxin contains 7 chains: A,C,D,E,F,G and H.Chains A and C belong to subunit A. Chains C,D,E,F,G and H belong to subunit B
Cholera toxin(CTX) has two types of subunits: subunit A and subunit B. A subunit contains A1 domain, which includes the enzymatic active site, and A2 domain, which has a α–helix tail. The B subunit contains five chains that form a pentameric ring around the central pore in structure; Subunit A and subunit B are assembled by the α–helix tail of A2 domain, which inserts into the central pore. CTX is the main virulence factor of the pathogen Vibrio cholerae and cause the major symptom of infection: extreme diarrhea, vomiting, cramps and even death [1],[2],[3].
The enzymatic subunit has a globular domain (CTA1) and a long helical domain (CTA2). Once the CTX binds to the cell surface, it is internalized, and its CTA1 domain binds to ADP-ribosylation factor 6(Arf6)[1], enabling its catalytic activity.
mRNA Structure
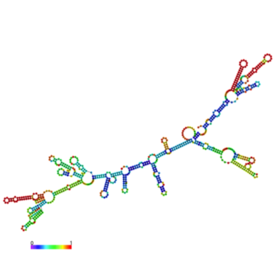
mRNA structure of CTX. The structure is predicted by the method of minimum free energy, using RNAfold WebServer.The colors represent the propensity of each nucleotide to participate in base pairs and whether a predicted base pair is well predicted. The scale ranges from red (highest probability) to blue-violet (lower probability).
RNA folding algorithm uses a method to minimize the free energy of the structure so that the RNA molecule is in the most stable form. The image at lower left is the predicted mRNA structure of CTX, which is made by RNAfold WebServer.
Function
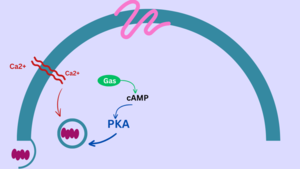
Cholera toxin, after being secreted from the Vibrio cholerae, binds to the enterocytes (intestinal cells) by the interaction between the subunit B and GM1 ganglioside[2] receptor on enterocytes, which then promotes the toxin endocytosis. Next, A1 turns to an active enzyme after separating with the A2 domain. After the A1 domain of subunit A of the toxin enters the cytosol, it activates adenylate cyclase to produce cAMP through G proteins, which triggers the activation of cystic fibrosis transmembrane conductance regulator (CFTR), leading to watery diarrhea: the efflux of water and ions from cells [4].
Evolution
CTXφ Bacteriophage which is carried by Vibrio cholerae produces Cholera toxin. Cholera toxin is encoded by the ctxA and ctxB genes that are introduced into V. cholerae strains by horizontal gene transfer [5].
Application
Subunit B of cholera toxin is designed to be applied as a neuronal tracer due to its non-toxic characteristic. It also used to identify lipid rafts as florescent tag on the cell surface since lipid rafts contains GM1 gangliosides, which will interact with subunit B during mechanism [6]. It is demonstrated that cholera toxin subunit B is a sensitive retrograde tracer for the central nervous system [7].