Background
Human G-protein coupled receptor 40 (hGPR40), also known as free fatty acid 1 receptor (FFAR1), is a seven helical transmembrane domain receptor for long-chain free fatty acids. hGPR40 stimulates insulin secretion.[1] Some known fatty acid substrates of hGPR40 include linoleic acid, oleic acid, eicosatrienoic acid, and palmitoleic acid[2]. This protein is primarily located in the pancreatic β-cells in the islets of Langerhans, and as such, it has become a target for potential Type 2 Diabetes treatments.[3] Type 2 diabetes is especially relevant because the cells have become desensitized to insulin and free fatty acids. Activation of hGPR40 stimulates insulin secretion and decreases extracellular glucose concentration.[4] GPR40 is a member of a group of homologous GPCRs all located on chromosome 19q13.1 including GPCR41, 42, and 43.[5] Evidence exists that shows GPCR43 is involved in adipogeneis. GPCR41 was previously believed to participate in adipogenesis, but this was shown to be false.[6]
Structure
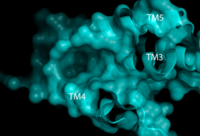
Figure 1. Second proposed binding site of hGPR40 with surface shown. Substrate would bind in the deep pocket shown between TM3, 4, and 5.
Like most G-protein coupled receptors, hGPR40 contains (). To obtain a crystallized structure of the protein, four (, , , ) were made to increase expression levels and thermal stability of the protein. These mutations did not significantly impact the enzyme's binding affinity with a known agonist, TAK-875.[1] A (shown in crimson) was also added to intracellular loop 3 to aid in the formation of crystals. T4 Lysozyme also had little effect on TAK-875 binding.[1] For clarity, lysozyme is removed in all further renderings of hGPR40.
Binding Sites
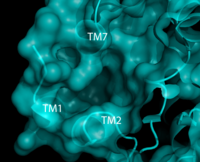
Figure 2. Third proposed binding site of hGPR40 with surface shown. Substrate would bind in the pocket below TM7 and above and inbetween TM 1 and 2.
identified multiple
binding sites in hGPR40.
[1] Full agonists and
partial agonists were shown to bind in separate sites with positive
cooperativity.
[7] The has been identified, but other binding sites were hypothesized. TAK-875 binds between transmembrane helices 3, 4, and 5 and underneath ECL2. By visual inspection, a second possible binding site was proposed between transmembrane helices 3, 4, and 5 on the intracellular side of the transmembrane helices (Figure 1). Also by visual inspection, a third possible binding site was proposed between transmembrane helices 1, 2, and 7 on the extracellular side of hGPR40, close to the TAK-875 binding site (Figure 2).
[1] These binding sites could potentially serve as regulation points for hGPR40. Many proteins are regulated by the binding of inhibitors.
Charge Network
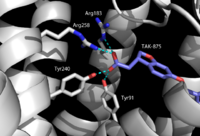
Figure 3. TAK-875 with key binding residues Tyr91, Arg183, Tyr240, and Arg258. These residues all hydrogen bond to the carboxylate moiety of TAK-875.
hGPR40 has a distinct binding pocket that is established by : Tyr91, Glu172, Arg183, Ser187, Tyr240, Asn241, Asn244, and Arg258. The importance of these residues for agonist binding was determined by alanine
mutagenesis studies. Each of these residues have either a
charged or polar R-group that allows them to develop a charge network. This network keeps the residues in a stable, unbound state until exposed to a substrate. When the substrate (an agonist) enters the binding pocket, four of the eight interact directly with the carboxylate moiety of the agonist. In 2007 and 2009, researchers showed the presence of Arg183 and Arg258 in the binding pocket.
[8][9] Along with the two arginine residues, the charge network incorporates two tyrosine residues (Figure 3). These residues (Tyr91 and Tyr240) also stabilize the carboxylate group on the agonists. It was further determined that Tyr240 is especially important for binding. Mutation of Tyr240 caused a reduction in the binding affinity of TAK-875 by eight fold and had a significant effect on the
KD of the protein.
[1]
ECL2
Although it may be different in many ways, hGPR40 is similar to most G protein coupled receptors because it contains a highly conserved hairpin loop. This extracellular loop (), is accompanied by a disulfide bond () and serves an important role in the protein. In hGPR40, ECL2 has two sections: a beta sheet and an auxiliary loop. The beta sheet (shown in cyan) spans helices 4 and 5. The ECL2 of hGPR40 differs from that of other proteins because it contains an auxiliary loop (magenta) of 13 extra residues. The entire extracellular loop has low mobility and flexibility which allows it to act as a cap for the binding pocket. The only exception to the low flexibility is the tip of the auxiliary loop, which corresponds to residues Asp152-Asn155. This area of greater mobility allows for substrates to enter the binding site.[1]
Function
hGPR40 functions as a free fatty acid receptor that participates in insulin signaling to regulate blood glucose concentrations. The actual mechanisms by which this occurs are unknown, but there are multiple theoretical mechanisms for how hGPR40 accomplishes this.
Mechanisms of Insulin Secretion
One proposed pathway of insulin secretion by hGPR40 involves the activation of the Gaq/11 protein complex. This complex then activates phospholipase C (PLC) which in turn hydrolyses phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 can then mediate the influx of Ca2+ by moving into the cytoplasm, binding to the endoplasmic reticulum, and allowing for the release of Ca2+ into the cytosol.[5] This increase in [Ca2+] amplifies the similar increase in [Ca2+] that results from high concentrations of glucose. In this way, hGPR40 mimics glucose dependent insulin secretion.[10] Overall, hGPR40 helps to amplify the Ca2+ signal so that the cell secretes more insulin.
Another pathway through which hGPR40 may induce insulin expression is through phospholipase D1 (PKD1). When free fatty acids bind to hGPR40, hGPR40 directly phosphorylates and activates PKD1. The PKD1 plays a role in controlling the organization of an actin network that plays in role in insulin secretion.[5]
Clinical Relevance
By signaling predominantly through Gaq/11, GPR40 increases intracellular calcium and activates phospholipases to generate diacylglycerols resulting in increased insulin secretion. Synthetic small-molecule agonists of GPR40 enhance insulin secretion in a glucose dependent manner in vitro and in vivo with a mechanism similar to that found with fatty acids. GPR40 agonists have shown efficacy in increasing insulin secretion and lowering blood glucose in rodent models of type 2 diabetes.[5]
TAK-875
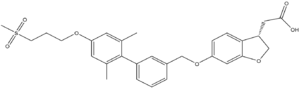
Figure 4. Structure of TAK-875. The carboxylate moiety (upper right) inserts into hGPR40. The Sulfonate group (upper left) remains on the outside of the protein once bound.
One example of an hGPR40 agonist is . The carboxylate moiety of the agonist enters through the auxiliary loop of hGPR40, disrupts the hydrogen bonding of the charge network, and binds with Arg183, Arg258, Tyr91, and Tyr240.
[1] TAK-875 has shown efficacy in increasing insulin secretion and lowering blood glucose in rodent models of type 2 diabetes.
[5] This drug was studied in stage III clinical trials. It significantly reduced
HbA1c and fasting plasma glucose levels in Japanese patients with type 2 diabetes that was not controlled by diet and exercise. However, clinical trials were stopped shortly after this study because TAK-875 was suspected of causing liver damage.
[11]




