You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Location
The human gene NOS1, found on chromosome 12, encodes for a nitric oxide synthase which is ubiquitously expressed. Nitric oxide synthases consume L-arginine to produce nitric oxide. The chemical equation is shown below.
2 L-arginine + 3 NADPH + 4 O(2) = 2 L- citrulline + 2 nitric oxide + 3 NADP(+) + 4 H(2)O
Nitric oxide is used in a wide variety of processes. A few examples of molecular processes include heme binding, NADP binding, calcium signaling, and oxidation-reduction reactions. Biological examples include regulation of cardiac muscle contraction, blood coagulation, aging, and regulation of neurogenesis [3]. There are three known isoforms known for NOS1 in humans produced by alternative splicing, as well as four natural transcript variants for NOS1. The alternatively-spliced isoforms are reffered to as neuronal NOS (nNOS or NOS1), endothelial NOS (eNOS or NOS3), and cytokine-inducible NOS (iNOS or NOS2). Moderate expression of NOS1 has been observed in skeletal muscle, brain, testicular, lung, and kidney tissue. Low expression has been observed in heart, adrenal gland, and retinal tissues [4].
Function
NOS1 is a major component of the production of Nitric Oxide. Nitric Oxide targets NO receptors on G-cyclase and other Nitric Oxide specific receptors as well. NOS1 is an important component of synaptogenesis, long-term potentiation, neurotransmitter release and synaptic plasticity [5].NOS1 is coupled with N-methyl-D-Aspartate (NMDA) receptors in the post-synapse of cells, which allows these processes to occur. The NMDA receptor binds to PSD95, which than binds to NOS1. After the complex is formed, NDMDA receptor mediated CA2+ influx now regulates the amount of Nitric Oxide that is produced by NOS1 [6].
NOS1 also contributes in limiting oxidative stress in the myocardium, limits systolic and diastolic dysfunction and prevent a failing heart. NOS1 regulates the reuptake of Ca2+ in sarcoplamisic reticulum (SR). NOS1 is shown into the human coronary artery smooth cells and maintains of basal blood flow. NOS1 also controls the parasympathetic and sympathetic regulation of the heart[7]. NOS1 is important in all functions that help regulate the cardiac muscles[8]. The ability of Nitric Oxide to self regulate enables NOS1 to control all these major functions. In myocite relaxation, the Nitric Oxide produced by NOS1 facilitates SERCA to increase intracellular CA2+ because of the increase in PKA dependent PLN phosphorylation. NOS1 also regulates cardiac function by the Nitric Oxide produced targets that are not CA2+ dependent[9].
Disease
Nitric oxide (NO) is produced from one of three synthases present within the body: neuronal nitric oxide synthase 1 (NOS1), inducible nitric oxide synthase 2 (NOS2), and endothelial nitric oxide synthase 3 (NOS3) (Shinkai, et. al, 2002). Specifically, NOS1 has nine different first exons that lead to multiple NOS1 transcripts with different 5’-untranslated regions (Galimberti, et. al, 2008). An advantage of having multiple first exons that can be alternatively spliced and expressed is that NOS1 can be specific and specifically regulated for different tissues (Galimberti, et. al, 2008). A disadvantage to having multiple first exons is that there is a higher possibility of mutations, which would affect NO production and thus have an effect on the second messenger cyclic guanine monophosphate (cGMP) production (Shinkai, et. al, 2002). Furthermore, because NO is an oxyradical, overproduction caused by a mutation can lead to neural tissue damage (Shinkai, et. al, 2002). Numerous pathologies may arise from neural tissue damage, and one study suggested overproduction of NO that leads to such damage is possibly an influential factor in developing schizophrenia (Shinkai, et. al, 2002).
Single nucleotide polymorphisms (SNPs) and various lengths of tandem repeats within NOS1 have been linked in other disorders of the brain such as Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Out of three identified SNPs occurring in alternative exon 1c, only the SNP G-84A has a functional effect that decreases transcription levels (Galimberti, et. al, 2008). However, various lengths of tandem repeats present in the alternative exon 1f has been shown to be a potential factor in both Alzheimer’s and Parkinson’s diseases (Galimberti, et. al, 2008; Rife, et. al, 2009). Shorter tandem TG repeats are possibly associated with the development of Alzheimer’s disease, and longer tandem TG repeats are possibly associated with the development of Parkinson’s disease (Rife, et. al, 2009). Although schizophrenia, Alzheimer’s, and Parkinson’s diseases have genetic influences, mutations in NOS1 can be a risk indicator for developing these diseases (Shinkai, et. al, 2002; Galimberti, et. al, 2008; Rife, et. al, 2009).
Relevance
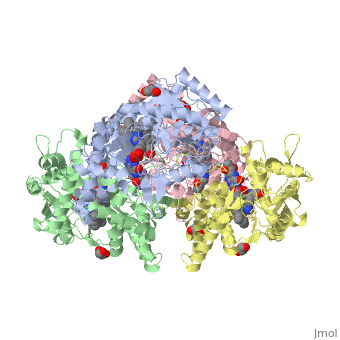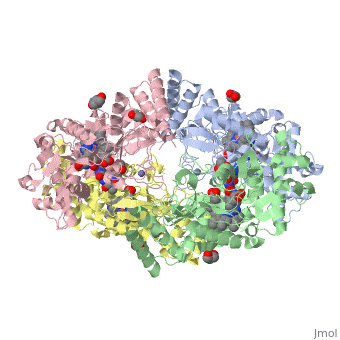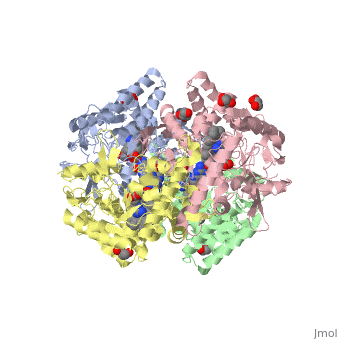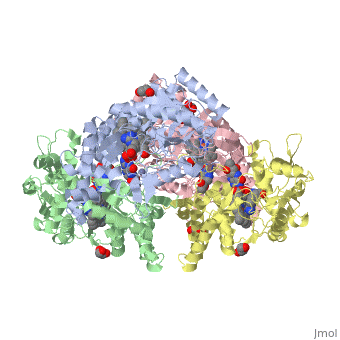
Structural highlights
Nos exists as a homodimer. This homodimer consists of two regions. One region is an N-terminal oxygenase domain and the other is a C-terminal reductase. The N-terminal oxygenase domain is an extended beta sheet cage with binding sites for heme and pterin. Nos has a N-terminal catalytic domain with a heme active site. Nos 1 also has a cofactor site nearby for tetrahydrobiopterin (H4B). Nos has a C-terminal reductase domain containing FMN, FAD and NADPH binding sites. Electrons are passed from FAD to FMN and then to the heme. This electron flow is controlled by the binding of CaM and Ca2+ in the linker region between the two major domains. The linker between the oxygenase and reductase domains contains a calmodulin-binding sequence. Nos1 is found in neuronal tissue.