Prinivil/Sandbox 1
From Proteopedia
Contents |
[1]Lisinopril
|
TEMP=Green links for structure TEMP=Needs Editing
Lisinopril (Commercially known as Prinivil) functions as a competitive inhibitor of the angiotensin converting enzyme (ACE). ACE cleaves specific residues of an inactive angiotensin 1 near the C domain (domain closer to the C-terminus) to form a potent vasopressor octapeptide known as angiotensin 2; however the specific substrate binding and catalysis is not fully understood. ACE is found as a type 1 membrane bound dipeptidyl carboxypeptidase that regulates blood pressure and steady state equilibrium of ions within the blood. Located on vascular epithelial cells, the drug interaction takes place on the surface of the cell within an artery/vein.[2] Lisinopril (prinivil) acts upon the membrane protein by forming tight and nonspecific contacts with the conserved residues in the S2’, S1’, and S1 positions of the ACE. The ligand is stabilized by 3 residues (2 histidines (RES #) and 1 glutamic acid (RES #)) We need to add the specific residue bindings here at some point interacting through hydrogen bonding along with an ionic binding of a Zinc atom and the carboxylate group which configures the molecule in 3D space.
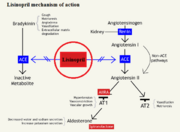
Drug Function
As seen in Figure 1, bradykinin will bind to the ACE, which allows for the conversion of angiotensin I to angiotensis II by theACE/bradykinin complex. By blocking the active site of ACE, an inactive angiostensin 1 cannot be cleaved, which would block the cascade of events triggered by angiotensin 2 binding to type 1 AT2 receptor to create vasoconstriction. ACE is also able to deactivate a common vasodilator known as bradykinin, but when bound to lisinopril, bradykinin levels rise in the blood stream causing a decrease in blood pressure and increased vasodilation.
Structure
Mechanism
Effect in Humans
TEMP
JSmol in Proteopedia [4] or to the article describing Jmol [5] to the re
</StructureSection>
References
- ↑ Canner, D. Lisinopril http://proteopedia.org/wiki/index.php/prinivil (accessed Nov 10, 2016).
- ↑ Natesh R, Schwager SL, Sturrock ED, Acharya KR. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature. 2003 Jan 30;421(6922):551-4. Epub 2003 Jan 19. PMID:12540854 doi:http://dx.doi.org/10.1038/nature01370
- ↑ Akif M, Georgiadis D, Mahajan A, Dive V, Sturrock ED, Isaac RE, Acharya KR. High-resolution crystal structures of Drosophila melanogaster angiotensin-converting enzyme in complex with novel inhibitors and antihypertensive drugs. J Mol Biol. 2010 Jul 16;400(3):502-17. Epub 2010 May 19. PMID:20488190 doi:10.1016/j.jmb.2010.05.024
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644