Sandbox1996
From Proteopedia
Diuril (Chlorothiazide)
| |||||||||||
References
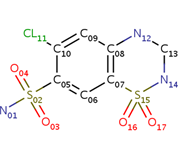
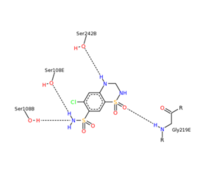
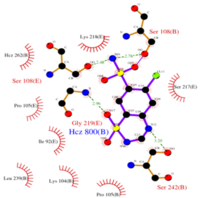
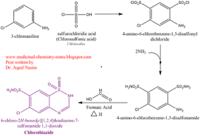
- ↑ 7</sub>H8ClN3O4S2 and a molecular weight of 298 Da. This chemical compound consists of an aromatic ring, benzothiadiazine, a sulfonamide group, and a chloride. It has a melting point of 272 degrees Celsius, a flash po int of 302.7 degrees Celsius, a solubility of 60 mg/ml in DMSO and less than 1 mg/ml in water, and appears as a white crystalline powder(reference). The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms (reference). . The enzyme cave of glutamate receptor 2 contained that enabled binding of chlorothiazide. Binding involved hydrogen bonding between the (Figure 2 & 3). Synthesis of chlorothiazide occurs through the reaction between 3-chloroaniline, chlorosulfonic acid, and ammonia; and it is catalyzed by formic acid (Figure 4)(reference).
Function and Mechanism
Chlorothiazide has diuretic and anti-hypertensive properties. Chlorothiazide acts as a diuretic, which means that it inhibits the reabsorption of chloride. This occurs at the distal tubules via the sodium-chloride co-transporter. The result of this is an increased excretion of sodium, chlorine, and water. Chlorothiazide also inhibits sodium ion transport across the renal tubular epithelium through binding to the thiazide-sensitive sodium-chloride transporter. The result of this is an increase in the excretion of potassium using the sodium-potassium exchange system. More specifically, chlorothiazide targets which mediates sodium and chloride reabsorption in the nephron segment to enhance renal sodium reabsorption. As for the anti-hypertensive properties of chlorothiazide, the mechanism is not quite as known. It is thought that vasodilation is caused by the activation of calcium-activated potassium channels (KCa) and the inhibition of carbonic anhydrases. <ref> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 </li> <li id="cite_note-one-1">[[#cite_ref-one_1-0|↑]] The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 </li> <li id="cite_note-four-2">[[#cite_ref-four_2-0|↑]] Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794. </li> <li id="cite_note-five-3">↑ <sup>[[#cite_ref-five_3-0|4.0]]</sup> <sup>[[#cite_ref-five_3-1|4.1]]</sup> Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html </li> <li id="cite_note-six-4">[[#cite_ref-six_4-0|↑]] RxList Inc. (2017) Medical definition of diuretic, RxList: The Internet Drug Index. Retrieved from http://www.rxlist.com/script/main/art.asp?articlekey=7103 </li> <li id="cite_note-seven-5">[[#cite_ref-seven_5-0|↑]] Crawford, J.D., Kennedy, G.C., and Hill, L.E. (1960) Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series, N. Engl. J. Med. 262, 737-743. </li> <li id="cite_note-three-6">↑ <sup>[[#cite_ref-three_6-0|7.0]]</sup> <sup>[[#cite_ref-three_6-1|7.1]]</sup> Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones </li> <li id="cite_note-two-7">[[#cite_ref-two_7-0|↑]] AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html </li> <li id="cite_note-eight-8">[[#cite_ref-eight_8-0|↑]] The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224 </li>
<li id="cite_note-nine-9">[[#cite_ref-nine_9-0|↑]] Jaffe, M.O. and Kierland, R. R. (1958) purpura due to chlorothiazide (Diuril), J. Am. Med. Assoc. 168, 2264-2265. </li></ol></ref>