default</scene>'>
Function
Luciferase is a class of bioluminescent enzymes that are found in several luminescence organisms. The most studied form Luciferase within its class is found in the North American Firefly (Photinus pyralis). This protein catalyzed the reaction that produces the distinctive yellow flash seen in from the abdomen of the insect. Photinus pyralis is known to use this mechanism for mate attraction and defense. Firefly Luciferase is unique to its species and different forms within the luciferase class can be found in other invertebrates and bacteria.[1]. The most notable being the bioluminescent enzyme found in Click Beetles (Pyrophorus). This protein is one of the most studied and widely used luminescence enzymes having various applications in cell and molecular biology.
Luciferase catalyzes two reactions; both reactions use an adenylation reaction along with ATP. The bioluminescent pathway produces Oxyluciferin and a photon of light. The other pathways does not produce light and is known as the nonluminescent reaction. This mechanism utilizes luciferin and produces Luciferyl-CoA. This addiotion of a CoA has been shown to be related to Acyl-CoA synthase used in the activation of fatty acids for oxidation.
Origin
Luciferase is part of the acyl-adenylate/thioester-forming superfamily. Through its two step reaction it ultimately forms an acyl-adenylate intermediate which is esterified into CoA. This mechanism is similar to the other members in this class some of which include Fatty Acyl AMP Ligases, Fatty Acyl CoA Ligases, and Acetyl CoA Ligases. There is evidence that Luciferase has homology with the highly conserved enzymes used in lipid synthesis. [2] Recent studies show that the Gene sequencing of firefly luciferase along with other related preformed and compared to other homologs of the gene. Studies suggest that firefly luciferase gene has similarities to drosophilla fatty acyl-Coa genes.
Disease
Relevance
This protein has been utilized in various types of assays ranging from quantification of ATP and the rate of transcription within a cell.[3]. This molecule is especially unique due to the fact that is very efficient in producing a photon through this reaction. Luciferase is sensitive to small changes in substrate and is a optimal choice for quantification of gene expression. It has potential for further biological applications in the future. Luciferase is widely used as a luminescent reporter gene in a variety of assays. The Luciferase gene can be isolated from the firefly and be inserted into animal or bacterial cells to monitor gene expression.
Structural highlights
The structure of this protein comprises of two prominent domains. The larger one contains an N terminal distorted beta-barrel accompanied by alpha helices. The second and smaller unit is consist of a beta sheet and alpha helix complex [4]. The process of fluorescence is achieved through a two-step oxidation reaction involving the substrate Lucinferin accompanied with ATP, Magnesium and oxygen. The first step consist of using ATP-Mg in an Acylation reaction of the COOH group on Lucinferin producing a Luciferyl adenylate intermediate and a phosphate group. The second reaction uses oxygen to create an excited state of the molecule. The molecule then returns to its ground state emitting a photon of light (Conti et al., 1996).
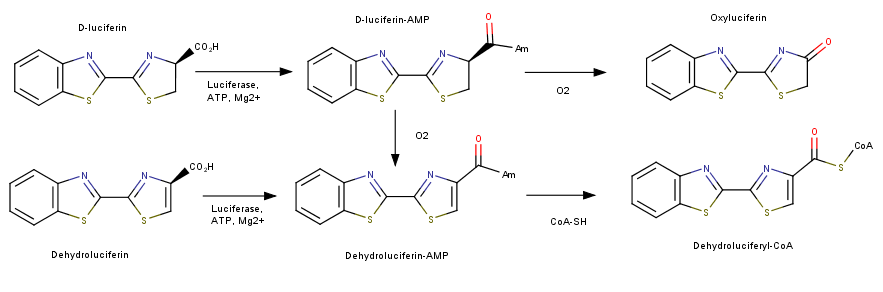
A single peptide has been discover that plays a vital role in the photooxidation by Luciferase. The specific amino acid is a histidine located in the region of the protein [5]. It has been shown to be necessary for the use of oxygen in the second part of the reaction.
Structure Specifications

