User:Harish Srinivas
From Proteopedia
|
Lipid Receptor S1P1 Activation Scheme
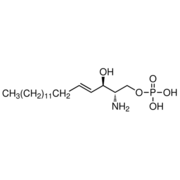
After binding of agonist S1P to the binding site of S1P1, the movement of acyl tail of S1P leads to the flipping of W2696.48 (step 1). Such rotameric change alters the conformation of side chain of F265 which is located next to W2696 in the same helix TM6 (step 2). These residues form a core of a transmission switch which involves rearrangement of centrally located residues including N63, D91, S304 and N307 They facilitate a redirected flow of water molecules inside a receptor (step 3). The influx of water molecules at intracellular part of the receptor leads to limited motions of cytoplasmic ends of TM helices, with the largest movement associated with TM7 (step 4), which is a prerequisite for larger motions of the cytoplasmic parts of transmembrane helices. These movements lead to opening the protein structure to make room for binding a G protein. The mutations of S1P1 receptor analyzed so far were located close to the orthosteric binding site of native agonist S1P.
Other Features
S1P1 phosphorylation on S351, a residue crucial for receptor internalization. Impaired S1P1 phosphorylation enhances TH17 polarization and exacerbates autoimmune neuroinflammation. S1P1 receptor internalization is a critical step in initiating S1P signaling. This process is dependent on post-translational modification of the C-terminal domain of the receptor. Binding of S1P to S1P1 promotes the phosphorylation of C-terminal domain serine residues of S1P1 by protein kinase GRK2. This covalent addition of phosphate residue modifies the physicochemical properties of S1P1 leading to internalization of the ligandreceptor complex.