Fumarase 2
From Proteopedia
Contents |
Fumarase
Overview
Fumarase, also known as fumarate hydratase, is a protein that functions as an enzyme in the metabolic pathway known as the Krebs cycle, or citric acid cycle. Fumarase includes only α-helices for its secondary structure, and it has four identical subunits in its biological state. In the seventh step of the reaction pathway, fumarase catalyzes the reversible reaction that converts fumarate to malate. A water molecule is used in the reaction to form malate; therefore, the mechanism of reaction involves hydration of fumarate in order to form malate. The kinetics demonstrated by fumarase catalyzing the reaction in both directions indicate that the forward pathway is energetically favored. However, fumarase activity can be regulated by allosteric effects or inhibition, and the allosteric effects mainly result from conformational changes when substrate binds to one of its sites.
Stucture
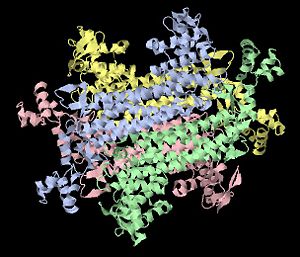
Fumarase is classified as an all alpha protein which belongs to the L-aspartase/fumarase family, and the enzyme specifically consists of four identical subunits which form a tetramer that appears symmetric(see image on right). From the four subunits, fumarase has three domains which comprise two binding sites: the active site and B site. Although the active site has a mostly solid structure and shifts very little when it binds to the substrate, the B site shifts substantially more upon binding, and this shift helps regulate affinity for molecule binding at the active site [1]. This has several implications for regulation of fumarase's activity and affinity to bind at the active site, but water molecules also play an important role in its function as an enzyme.
Mechanism of Reaction
Fumarase functions as an enzyme in the citric acid cycle responsible for catalyzing the reversible reaction involving water addition to fumarate in order to form malate. The reaction mechanism consists of two main steps and requires a water molecule because it reacts via hydration. The first step of the reaction involves addition of a hydroxy group from the water molecule to a double-bonded carbon in fumarate. When the hydroxy ion bonds with a carbon, an electron from the double bond moves to the other carbon atom which forms a carbanion transition state. Finally, a proton from the water molecule bonds to the carbanion, forming malate [2]. In the , amino acid residues involved in binding the substrate are located on three subunits: Thr100, Ser139, Ser140, and Asn141 from the b-subunit, Thr187 and His188 on the d-subunit, and Lys324 and Asn326 of the c-subunit [3]. The B site is located in a π-helix turn between the active site and solvent, and it includes residues Arg126, Lys127, Val128, His129, Pro130, Asn131, and Asp132 all on the b-subunit. Two hydrogen bonds initiate the binding of Asn131 and Asp132 residues with S-malate.
|
Kinetics
Fumarase catalyzes the reversible reaction between fumarate and malate in the citric acid cycle of cellular metabolism. When it catalyzes the addition of water to fumarate in order to form malate, the Km and Vmax values are 0.30 mM and 129 s-1, respectively. The reverse reaction (dehydration of malate to form fumarate) has a Km of 0.10 mM and Vmax of 60 s-1 [4]. Thus, the forward pathway is favored because of its higher Km and Vmax values which means that converting fumarate to malate is more energetically favorable. Fumarase kinetics normally follows Michaelis-Menten kinetic plots at low concentrations of substrate, but high substrate concentrations influence the enzyme’s activity due to allosteric effects. Enzyme kinetics studies with fumarase mutants differ from wild type fumarase kinetics when the mutations alter amino acid residues involved in binding at the active site and B site. Also, the Michaelis-Menten kinetics plots for fumarase mutants exhibit a sigmoidal curve which suggests the presence of cooperativity in the enzymes activity.
Regulation
Similar to most enzymes involved in biological processes, fumarase can be regulated in several different ways. Allosteric effects commonly regulate fumarase activity via substrate and inhibitor binding with the active site. Fumarase activity is both positively and negatively regulated by substrate concentration. When the concentration of a substrate is five-fold of the Km value, it activates fumarase’s activity; however, substrate concentrations above 0.1 M result in inhibition of fumarase function [3]. The concentration of substrate influences cooperativity of fumarase, depending on the availability of substrate to bind to domains.
The regulation of fumarase via allosteric effects involves conformational changes that occur when a substrate binds to the active site. Studies involving amino acid residue manipulation in the B site show that the B site helps regulate the binding affinity for the active site by allosteric effects [3],[4]. According to Weaver (2004), the active site and B site are located 12 Å apart which suggests that the conformational changes resulting from with the B site influence the active site affinity to bind with the substrate [1]. Inhibitors also regulate the activity of an enzyme via binding to the active site. Both citrate and succinate are known as competitive inhibitors of fumarase since they negatively influence the enzyme’s activity. They are competitive inhibitors because they have structural similarity to the substrate; therefore, the inhibitors compete with substrates to bind with the active site. The natural state of fumarase commonly involves with the active site in which similar amino acid residues responsible for binding with a substrate result in binding with a citrate molecule.
Other Interesting Information
Fumarase expression mainly occurs in skin, parathyroid, lymph, and colon tissues, and it is present throughout all life stages, from early development to mature adults. Fumarase comprises two specific classes which relate to the enzyme's: arrangement of subunits, metal ion requirement, and thermal stability. Class I fumarase isozymes can change their state, become inactive upon exposure to heat or radiation, are sensitive to superoxide anions, and Fe2+ dependent. Class II includes fumarase found in eukaryotes and prokaryotes, and they are iron-independent and thermally stable. Mutations in the gene that encodes fumarase can lead to a deficiency in fumarase enzyme in the citric acid cycle which is known to cause certain diseases. Autosomal recessive mutants can result in fumarase deficiency, a metabolic disorder distinguished by excess fumarate in the body which can lead to severe developmental defects [5]. Inheritance of this autosomal recessive mutation has serious effects on early neural and brain development and can be fatal. Also, heterozygous fumarase mutations play a role in cancerous tumor development; specifically, the mutant H153R has identified as a factor in three families of malignant tumor growths [6].
References
- ↑ 1.0 1.1 Weaver,T. Structure of free fumarase C from Escherichia coli. Acta Crystallographica (2005), D61, 1395-1401. [http://dx.doi.org/10.1107/S0907444905024194 doi:10.1107/S0907444905024194]
- ↑ Voet, D., Voet, J. & Pratt, C. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd Ed. NJ: John Wiley & Sons, Inc., (2008), 583.
- ↑ 3.0 3.1 3.2 Beeckmans, S. & Van Driessche, E. Pig heart fumarase contains two distinct substrate-binding sites differing in affinity. Journal of Biological Chemistry (1998), 273(48), 31661-31669.
- ↑ 4.0 4.1 Rose, I. & Weaver, T. The role of the allosteric B site in the fumarase reaction. Proc. Natl. Acad. Sci. USA (2004), 101(10), 3393-3397. [http://www.pnas.org/cgi/doi/10.1073/pnas.0307524101 doi:10.1073/pnas.0307524101]
- ↑ Remes, A., Rantala, H., Hiltunin, K., Leisti, J. & Ruokonen, A. Fumarase deficiency: two siblings with enlarged cerebral ventricles and polyhydramnios in utero. Pediatrics (1992), 89(4), 730-734.
- ↑ Kokko, A., Ylisaukko-Oja, S., Kiuru, M., Takatalo, M., Salmikangas, P., Tuimala, J., et al. Modeling tumor predisposing FH mutations in yeast: effects on fumarase activity, growth phenotype and gene expression profile. Int. J. Cancer (2006), 118(6), 1340-1345.