Protein 5c65 is part of the protein class GLUT. The GLUT proteins class facilitates the transport of glucose and hexoses over the plasma membrane is highly evolutionarily conserved across phyla. It is important to note that this protein is an isoform of GLUT 1, a protein which mediates equilibrative glucose transport. Specifically, the GLUT3 protein is mostly expressed in neurons, where it is believed to be the primary glucose transporter. In certain cases, it is expressed to facilitate glucose transport in the placenta. This protein was isolated from human liver cDNA libraries.
Function
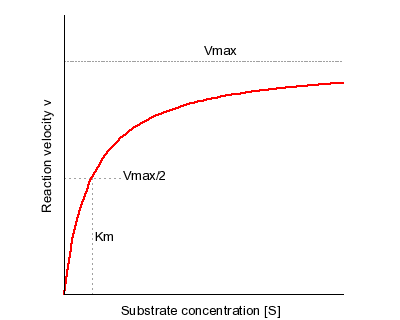
The primary biological function of this protein is for glucose transmembrane transport, or the transport of glucose molecules over the plasma membrane. Using the Michaelis-Mentin Kinetics Model, the GLUT3 mediated transport of glucose had a higher affinity during the outward substrate binding site. Secondly, the transport occurs more efficiently and quickly when the substrate is present on the trans site of the membrane.
Disease
Current research has made insights in to the structure of GLUT3, allowing for investigations into variable expression resulting in association with disease processes. Certain GLUT proteins, such as GLUT1 and GLUT3, has increased expression in cancer cells. Analyzing the variation in expression patterns within this protein has been used as a diagnostic tool, and in certain cases it can be used as an imaging tool in oncology. Lastly, there are currently investigations into the applications of GLUT as a drug delivery mechanism due to its binding and conformation properties.
Relevance
Both the amino and carbonyl termini of the protein are exposed on the cytoplasmic side of the plasma membrane. This protein functions via the alternative confirmation model. A transport protein exposes a substrate towards either the outside or inside the cell. When the glucose or hexose binds to the site, it catalyzes a conformational change, releasing the glucose on the other side of the membrane. GLUT3 is a unique glucose transporter in that it functions even in times of low glucose.
Structural highlights
The protein contains and has no known post-translational modifications. The first 6 transmembrane helices are in a pseudo symmetrical configuration relative to the last 6 helices. Helices 1, 2, 4, 5, 7, 8, 10, and 11 form an inner bundle that is stabilized by the outer helices 3, 6, 9, and 12. The GLUT3 protein is comprised of ~500 amino acid residues. It has a single site for N-Linked glycosylation, a central cytoplasmic linker domain, and exhibit topologies with their N and C termini, which are both positioned in the cytoplasm.
The protein also contains nine . The molecule itself is octyl glucose neopentyl glycol.
The protein has another kind of ligand, . This molecule is cholesterol hemisuccinate.