User:Mark Macbeth/Sandbox2
From Proteopedia
Contents |
Background
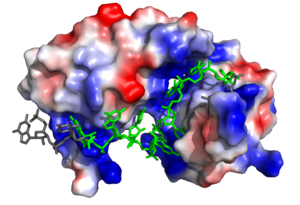
Sex Lethal Protein (Sxl) is a splicing repressor in the male developmental pathway of sex determination of the common fruit fly, Drosophila melanogaster[1]. regulates alternative splicing pathways to promote the expression of female sex-linked proteins. In eukaryotes, splicing is carried out via the spliceosome, a ribozyme-protein complex which binds to the 5’ and 3’ splice sites. As Sxl is a splicing repressor, it prevents the binding of the U2AF and U1 subunits of the spliceosome at their respective splice sites, which represses the alternative splicing mechanism[2]. As a result, the fruit fly expressing Sxl will produce mRNA transcripts encoding proteins for the female developmental pathway[1].
Significance
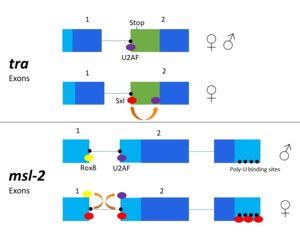
The Sxl RNA splicing targets encode for the transformer (tra) and the male-sex lethal (msl-2) proteins. Tra is a splicing activator for the female developmental pathway, and msl-2 modulates X chromosome application in male fruit flies. The mechanism for how Sxl targets these pathways differs slightly. In both mechanisms, Sxl occupies the 3' splice site and prevents U2AF from binding. This causes the U2AF splicing factor to bind at a downstream splice site encoding proteins in the female developmental pathway. In msl-2 targeting, Sxl also blocks the binding of another regulatory splicing factor, Rox8, and the U1 snRNP at the 5’ splice site[2]. Sxl can also control its own splicing pattern to conserve female expression. It does so by binding to Exon 3 of its own RNA and creating an RNP complex to eliminate this exon. After removal of Exon 3, Sxl becomes active and female expression is maintained.
| |||||||||||
Additional Reading
For more information on the U2AF splicing factor.
Relevance
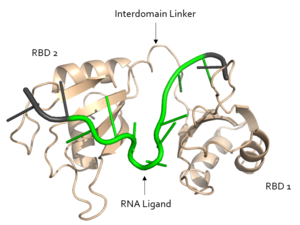
As Sxl functions as a splicing repressor, it may give insight into the effects of varying mechanisms of alternate splicing both in flies and other species. Sxl may also lead to understanding of human alternative splicing factors. As an RNA binding protein, research regarding Sxl may contribute to the understanding of enzymes with RNA recognition motifs. The Sxl RNP motif of RBD1 is also conserved in the ELAV family of proteins[1].
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 Handa N, Nureki O, Kurimoto K, Kim I, Sakamoto H, Shimura Y, Muto Y, Yokoyama S. Structural basis for recognition of the tra mRNA precursor by the Sex-lethal protein. Nature. 1999 Apr 15;398(6728):579-85. PMID:10217141 doi:10.1038/19242
- ↑ 2.0 2.1 2.2 2.3 Penalva LO, Sanchez L. RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol Mol Biol Rev. 2003 Sep;67(3):343-59, table of contents. PMID:12966139
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291-336. doi: 10.1146/annurev.biochem.72.121801.161720., Epub 2003 Feb 27. PMID:12626338 doi:http://dx.doi.org/10.1146/annurev.biochem.72.121801.161720