Alpha-Galactosidase is an enzyme found in many different lifeforms. Enzyme information can be found at
Function
a-Galactosidases catalyze the hydrolytic cleavage of the terminal a-galactose residue from many oligosaccharides and polysaccharides.[1] Although humans have lysosomal a-Galactosidase, the enzyme is not generally present in the human digestive tract; as a result, foods that contain raffinose, a carbohydrate broken down by a-Galactosidase, are not digested and passed through the upper intestine until they reach the lower intestine. There, a-Galactosidase producing bacteria finish the breakdown of it. Foods that contain raffinose include certain vegetables and legumes, such as beans. When the bacteria in the lower intestine ferment the carbohydrate, they release gases that can lead to flatulence. Beano is an over-the-counter supplement that contains a-Galactosidase and is promoted to prevent bloating when taken with meals containing raffinose. The enzyme in Beano is isolated from Aspergillus niger.
Mechanism
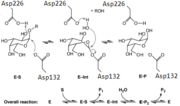
This a-Galactosidase, along with Human a-Galactosidase, reacts via a double displacement mechanism. Asp132 acts as the nucleophile while Asp226 functions as the acid/base catalyst.[2] a-Galactosidase is part of a group of enzymes known as glycoside hydrolases, which generally have 1 of 2 mechanisms. The 1st mechanism is a 1 step mechanism that induces the inversion of the stereochemistry of the substrate anomeric center, while the 2nd mechanism is a 2 step mechanism that preserves the stereochemistry. a-Galactosidase uses the 2-step mechanism.
Disease
Defects in the human a-Galactosidase gene can cause Fabry disease, a lysosomal storage disorder involving buildup of a-galactosylated substrates in tissues. It is an X-linked inherited disorder that affects 1 in 40,000 males. It is often characterized by chronic pain, vascular degeneration, cardiac anomalies, as well as other symptoms. The disease can show different levels of severity correlated with the amount of residual enzymatic activity. Organs affected can include the eyes, liver, kidney, and heart. More severe forms generally results from a complete loss of enzymatic activity, while milder phenotypes typically show some enzyme activity. Most people suffering from Fabry disease have a single point mutation in the gene, and over 400 missense and nonsense mutations have been identified. Other lysosomal storage disorder include the inherited diseases Tay-Sachs, Sandhoff, and Gaucher diseases.
Relevance
These enzymes are found in a variety of organisms, including bacteria, fungi, plants, and animals. They often function as digestive enzymes.
Structural highlights
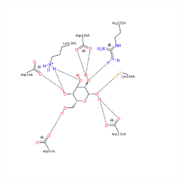
This shows the carbohydrate Galactose in the pocket of the active site. The , Asp132 and Asp226, can be seen sandwiching the inhibitor and product of this enzyme.
It is also interesting to note that all 5 hydroxyl groups participate in hydrogen bonding with enzyme residues. Only 5 residues are shown in , but there are more.
The structure of the enzyme contains comprised of 17 monosaccharides (15 monosaccharides while complexed with galactose). It also contains 2 domains, an that can be described as having an a/B barrel topology, as well as a that has an anti-parallel B-structure.