Function/Mechanism
Threonyl t-RNA Synthetase or
Threonyl-tRNA ligase or
TARS is class II
Aminoacyl-tRNA synthetase enzymes. These enzymes primary function are to added the respective amino acid to the respective transfer Ribonucleic Acid (tRNA-AA) The main function of the enzyme is to add Threonine amino acid (Thr) to threonine specific tRNA (tRNA-thr) a necessity prep for the protein synthesis pathway. The structure to th Below displays the overview of the Aminoacylation rxn.
[1]
Overall TARS protein rxn. Substrates includes Adenosine triphosphate (ATP), Threonine (Thr) and threonine specific transfer Ribonucleic Acid (tRNA-thr). TARS adds amino acid to tRNA by a two-step mechanism. First the enzyme binds to both and in the catalytic domain to perform an adenylation reaction in which pyrophosphate is released as a byproduct. This is then follow up by a transferring Thr from Adenosine monophosphate molecule to 3'OH site of tRNA-thr. [2] Image below demonstrates the arrow pushing occuring to generate threonine bound tRNA-thr.
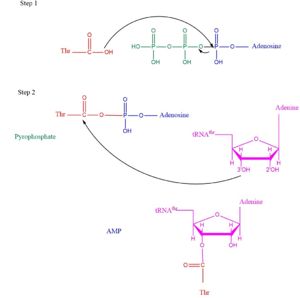
Arrow pushing of Aminoacylation rxn.[3] Structural highlights
Evolutionarily related proteins
List to available structures
References
- ↑ Rajendran V, Kalita P, Shukla H, Kumar A, Tripathi T. Aminoacyl-tRNA synthetases: Structure, function, and drug discovery. Int J Biol Macromol. 2018 May;111:400-414. doi: 10.1016/j.ijbiomac.2017.12.157., Epub 2018 Jan 3. PMID:29305884 doi:http://dx.doi.org/10.1016/j.ijbiomac.2017.12.157
- ↑ Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- ↑ Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.