User:Khadar Abdi/Sandbox1
From Proteopedia
Threonyl-tRNA Synthetase/ligase
General Function/MechanismThreonyl t-RNA Synthetase or Threonyl-tRNA ligase or TARS is class II Aminoacyl-tRNA synthetase enzymes. These enzymes primary function are to added the respective amino acid to the respective transfer Ribonucleic Acid (tRNA-AA) The main function of TARS is to add Threonine amino acid (Thr) to threonine specific tRNA (tRNA-thr) a necessity prep for the protein synthesis pathway. Below displays the overview of the Aminoacylation rxn. [1]TARS adds amino acid to tRNA by a two-step mechanism. First the enzyme binds to both and in the catalytic domain to perform an adenylation reaction in which pyrophosphate is released as a byproduct. The image to the right displays the binding of adenylate product to the TARS enzyme (PDB entry 1nyq). This is then follow up by a transferring Thr from Adenosine monophosphate molecule to 3'OH site of tRNA-thr. [2] The image below demonstrates the arrow pushing occurring to generate threonine bound tRNA-thr. 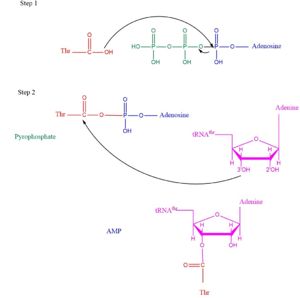 Arrow pushing of Aminoacylation rxn.[3] Lately the aaRS family was found to have more function than just aminoacylation. For instance, many aaRs molecules have been found to link with angiogenesis, blood vessel growth occuring within a cancer environment [4]. This is seen in human exogenous TARS in its ability to generate blood vessels within ovarian cancer environment [5]. Studies on the structure of TARS bound to BC194, derivative to the natural antibiotic Borrelidin, were investigated to understand how the angiogenic signaling from TARS occurs. Structural highlightsEvolutionary related proteinsList to available structuresReferences
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
| ||||||||||||

