4Q7Q is a protein found in Chitinophaga pinensis, a soil bacterium in the sphingobacterial family. Its structure has been previously characterized and exists in Protein Data Bank. Its function, however, has not.
After structural and sequential analysis via various databases including BLAST, Pfam, Dali, PyMOL, and ProMOL, we initially predicted that 4Q7Q is a hydrolase. More specifically, we hypothesized that it was a lipase, an enzyme that can hydrolyze lipids to form fatty acids and a glycerol molecule.
4Q7Q's Structure
4Q7Q is composed of two chains; one chain can be seen . The colors indicate the translation direction of the peptide sequence from the N to C terminus; red represents the N-terminus while dark blue represents the C-terminus. Based on this structural model, we can see that 4Q7Q is an alpha-beta superfold; there are beta sheets (represented by the straighter strands) sandwiched between the alpha helices (represented by the coiled strands).
In silico Analysis
We initially analyzed 4Q7Q through the protein structure databases BLAST, Pfam, and Dali. Our top hit was 4M8K, a GDSL-like lipase. Through BLAST, we found that 4M8K and 4Q7Q had a 36% sequence identity, with an E value of 0.002, indicating that it is a significant match. Since we can use the principle of homology to predict the function of an unknown protein, we first hypothesized that 4Q7Q was too a lipase.
Through analyzing the sequence of 4Q7Q in SnapGene and then analyzing the 3D structure in PyMOL, we hypothesized that a possible catalytic triad of 4Q7Q was Ser164, Asp193, and His196. We believe that this group of amino acids may be involved in active site of 4Q7Q and therefore affects how the protein works. As seen in this , all three amino acids are close in proximity to one another and are brought together in a single orientation.
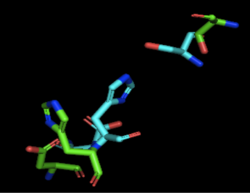
Figure 1: Alignment of 4Q7Q's putative catalytic triad and 3LIP's catalytic triad: Asp264, Ser87, His286. 4Q7Q is shown in green and 3LIP is shown in blue. The RMS is 2.257.
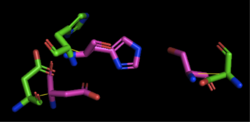
Figure 2: Alignment of 4Q7Q's putative catalytic triad and 1TAH's catalytic triad: Asp263, Ser87, His285. 4Q7Q is shown in green and 1TAH is shown in pink. The RMS is 2.205.
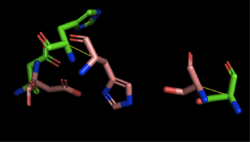
Figure 3: Alignment of 4Q7Q's putative catalytic triad and 1BWR's catalytic triad: Asp192, Ser47, His195. 4Q7Q is shown in green and 1BWR is shown in pink. The RMS is 2.049.
We also performed further analysis in PyMOL and ProMOL which involved the homology of active sites. Top hits included 3LIP, a lipase found in
Burkholderia cepacia, 1TAH, a lipase found in
Burkholderia glumae, and 1BWR, a hydrolase found in
Bos taurus. We aligned putative catalytic triad of 4Q7Q with each of the catalytic triads of these known proteins.
3LIP is a two chained protein. When aligning the catalytic triad of 3LIP using the Catalytic Site Atlas to the putative catalytic triad of 4Q7Q, the RMS is 2.257.
1TAH has four chains. When aligning the catalytic triad of 1TAH to the putative catalytic triad of 4Q7Q, the RMS is 2.205.
1BWR has one chain. When aligning the catalytic triad of 1BWR to the putative catalytic triad of 4Q7Q, the RMS is 2.049.
Compiling all of the data together, we can see that 1BWR is most structurally similar to 4Q7Q due to the low RMS values calculated when aligning both protein structures and catalytic triads. Therefore, we hypothesized that 4Q7Q is most likely a hydrolase; through experiments, we can investigate further if 4Q7Q is specifically a lipase.
Bacterial Transformation and Plasmid Purification
Before characterizing the function of 4Q7Q, we first needed to synthesize the protein through first transcribing 4Q7Q's DNA to amplify it and then translating it to express it. First, 4Q7Q's DNA was transcribed using its expression vector, the plasmid pMCS573. Since transformation must occur within a cell, the plasmid was transformed into DH5a cells using protocol from New England Biolabs.
After transformation, DH5a cells were lysed and spread on plates containing LB and ampicillin. Ampicillin was used because 4Q7Q's plasmid is ampicillin resistant. Therefore, only the bacteria that have been transformed with 4Q7Q's plasmid will grow on the plates. 4Q7Q's plasmid was then purified using Zyppy Plasmid Miniprep Kit.
However, although DH5a cells maximize the efficiency of transformations, they do not contain T7 polymerase, which is essential for protein expression. Therefore, the purified plasmid underwent another bacterial transformation into BL21 (DE3) cells that do contain T7 polymerase using protocol from New England Biolabs.
Protein Expression
Protein Purification
pNPB Lipase Assay