User:Luis Netto/Sandbox 1
From Proteopedia
Contents |
Ohr
| |||||||||||
It is involved in the response of
Disease
related with relevance
Relevance
Oxidants such as fatty acid hydroperoxides are signaling molecules involved in host-pathogen interactions, and therefore, their levels are strictly controlled by peroxidases and other mechanisms [1±4]. Ohr (Organic hydroperoxide resistance) proteins are Cys-based, dithiol-dependent peroxidases that display unique biochemical and structural properties [5,6]. Ohr enzymes play central roles in the bacterial response to peroxynitrite and fatty acid hydroperoxides, two oxidants involved in host±pathogen interactions [1]. These enzymes are found in bacteria and fungi, and they are absent in their hosts (plants and animals) [7], making them promising targets for drug discovery. Some examples of pathogenic bacteria that express Ohr proteins are Pseudomonas aeruginosa, Vibrio cholerae and Xyllela fastidiosa [7]. Xylella fastidiosa is a plant pathogen with agronomic interest, causing disease in citrus, grapes and olives [8].
Structural highlights
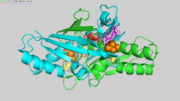
Ohr is a homo-dimer, with a symmetrical,oval shape. The two monomers are tightly wrapped around each other in a head-to-tail orientation to generate a compact quaternary structure.
Arg19 and Glu 50 of one chain (lets say chain A) compose the triad with Cys61 of the other chain (in this case chain B).
References
- ↑ Cussiol JR, Alves SV, de Oliveira MA, Netto LE. Organic hydroperoxide resistance gene encodes a thiol-dependent peroxidase. J Biol Chem. 2003 Mar 28;278(13):11570-8. doi: 10.1074/jbc.M300252200. Epub 2003 , Jan 22. PMID:12540833 doi:http://dx.doi.org/10.1074/jbc.M300252200
- ↑ Lesniak J, Barton WA, Nikolov DB. Structural and functional characterization of the Pseudomonas hydroperoxide resistance protein Ohr. EMBO J. 2002 Dec 16;21(24):6649-59. PMID:12485986
- ↑ Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1998 May;180(10):2636-43. PMID:9573147