Hfq is a bacterial cytoplasmatic protein, first named for being a Host Factor for the Replication of the Qβ Phage RNA[1 - Schumacher 2002]. Hfq acts pleiotropically and impacts both the degradation and the translation efficiency of mRNAs [2 - Azam 2000], through the modification of sRNAs[3 - Wassarman 2001]. Besides, Hfq has a strongly conserved structure[4 - Kajitani 1994] and belongs to the LSm protein family, present in each of the three domains of life. Despite Hfq’s underscored importance, there is a wide spectrum of cellular functions, as growth rate and cell length, that are directly dependent of its chaperone function and have been discovered in the last century[5,6,7,8 - Artigos baixados], but many others remain unknown.
Structure
is a doughnut shaped homohexamer of roughly 8,9kDa, ~65Å diameter and ~23Å width, as described in S.aureus[1]. The action mechanisms of Hfq as a chaperone are based in its interaction with sRNAs. The best literature described functions among them are: the sequestering of ribosome binding sites (RBS); the inhibition of RBS-blocking mRNA regions, allowing translation; the stabilization of sRNAs which would otherwise be degraded; and the degradation of mRNAs. Hfq might also contribute to the degradation of certain mRNAs directly, but rarely. [9]
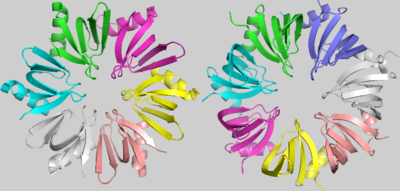
Hfq (Left) and SmAP1 (Right)
Function
Disease
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue. The Hfq protein is composed in its secondary structure.

