Proteopedia:Featured JRN/1
From Proteopedia
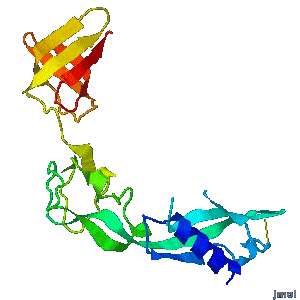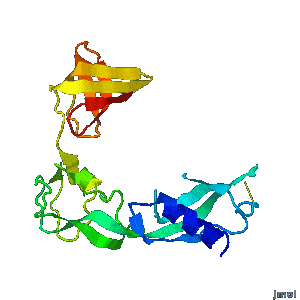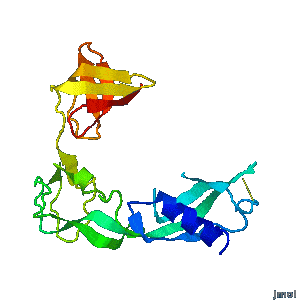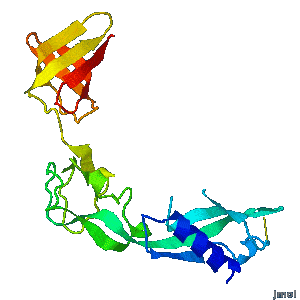 |
Structural flexibility of the periplasmic protein, FlgA, regulates flagellar P-ring assembly in Salmonella enterica.
Hideyuki Matsunami, Young-Ho Yoon, Vladimir Meshcheryakov, Keiichi Namba, and Fadel A. Samatey >>> Visit this I3DC complement >>> |
