Structural components of the B2 GPCR.

Disulfide Linkages between the TMD and ECL
Venus fly-trap position of the binding pocket
Amini and Carboxyl terminal groups
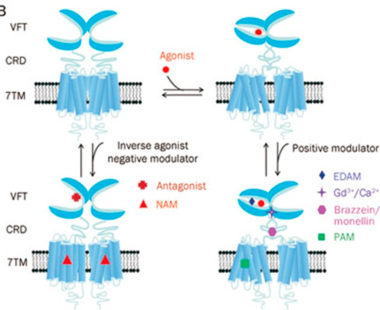
G-Protein Coupled Receptors (GPCR), also known as 7 transmembrane receptors [because of its 7 constituent alpha helices] are the largest groups of transmembrane protein receptors in eukaryotes. There are many different classes GPCRs depending on the position of their binding sites or the kind of ligands they bind to. There are about six classes of GPCRs and they include the classes A, B, C, D, E, AND F. Beta adrenergic receptors fall the class C with other metabotropic hormones while Rhodopsin (the light sensing cells in our eye) are categorized under class A. All studied GPCRs have a conserved structure that can be subdivided into; the extracellular domain (ECD), which comprises the three extracellular loops (EC1), (EC2), (EC3) and the N-terminal amino groups, the seven spanning helices transmembrane domain (7TM) embedded within the plasma membrane, and the intracellular domains ICD which also comprises the carboxyl-terminal residues and three intracellular loops (ICL1, ICL2, ICL3). The binding of the receptor to a ligand causes a structural change in the intracellular domains that also signals/activates the alpha subunit of a G-protein to exchange GDP for GTP, transducing the signal further downstream.
For some GPCRs, the ligand binds to a site in the 7TM (certain class A) or both the ECD and the 7TM as in certain class B GPCRs. However, in the Beta Adrenergic receptor, the ligand binds at the the extracellular domain [ECD] which acts like a fly-trap pocket. The transmembrane domain is structurally coupled to the extracellular domain through Disulfide linkages and mutations within this region disrupts the entire structure and function of GPCRs.
The intra cellular domain sits in the cytoplasmic end of the transmembrane domain. This region contains the D/E R Y motif; a conserved region within the ICD that interacts with interacts with intracellular ligands like the G-subunit (activates it) and phosphorylate other kinases as well. The C-terminal domain is structurally independent of the ECD and can adopt functionally discrete conformation in the absence of transmembrane helices. The C-terminal domain regulates the interactions with cytosolic proteins involved in signaling.
FUNCTIONS
GPCRs act as a bridge and communicate the conditions of the cell to the nucleus to induce transcription or affect the processivity of catalytic pathway by activating certain enzymes. They bind to wide varieties of ligands such as epinephrine, glucagon, insulin, light, and sugars. Upon binding the ligand epinephrine, it causes a conformational change that leads to the exchange of the Guanine nucleotide (GDP to GTP) and that mechanism "turns" the switch on. The apha subunit then dissociate from the beta and gamma subunits and activates adenylate cyclase which makes cAMP. The cAMP activates Protein Kinase A by binding to its regulatory subunits, and that releases the catalytic subunits to further phosphorylate other proteins in the cell.
GPCRs control many activities in the cell including the dopamine pathway [secretes the hormone dopamine; which is involved in reward, learning], the rhodopsin pathway [involved in vision], etc. All these pathways play vital roles and are very crucial for survival.
ENERGETICS
Experimental studies have shown that GPCRs do trigger cellular activities in their unbound states at a minimum basal levels. Studies have also shown that even when bound to agonists, GPCRs express characteristics that range from inactive to a continuum of partially active structures in the absence of the G protein. This means that the presence of the agonist plays an important role in the activation of GPCRs.
The kind and specificity of the agonists determine the activation pathway taken by GPCRs in terms of the energy ordering of the different states which is also premeditated by the respective activation energy landscapes. This means that the agonist-bound inactive state will have the lowest energy state among the various states within the degree of activation.
The stabilizing factor of the activated state of the GPCRs is attributed to the interactions between the Ga (G alpha subunit) and the GPCR.
DISEASE
Many GPCRs have become drug targets of many pharmaceutical drugs because of their central role in controlling many metabolic activities in the cell. Diseases including cancer, diabetes, alzheimer's, sleep deprivation and many others are treated by exploiting the druggability of GPCRs and how they mediate their responses to hormones, cytokines, and neurotransmitters. Of all drugs on the market that have been
approved by the CDC, about 35% elicit their potency and mediate their effects through GPCRs.



