Sandbox Reserved 1481
From Proteopedia
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Crystal structure of the catalytic domains of Mettl3/Mettl14 complexInsert caption here
Drag the structure with the mouse to rotate
Insert caption here |
| Drag the structure with the mouse to rotate |
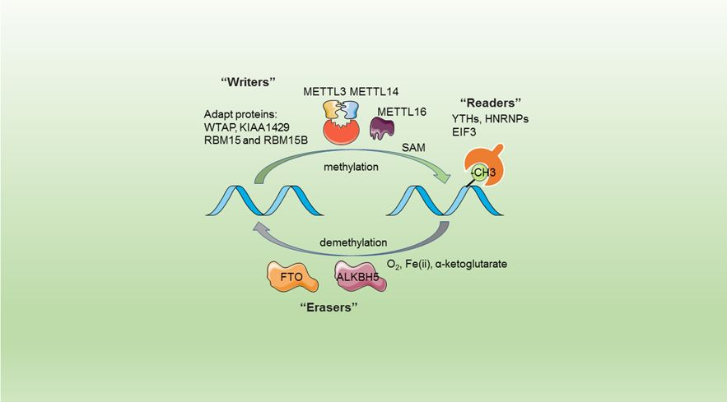
The complex METTL3/METTL14 is a heterodimer enzymatic complex involved into RNA post-transcription modifications by humans. This complex is abble to add a methyl group on adenosin of the RNA, by catalyzing a m6(A) modification.The N(6)-methyladenosine (m(6)A) is a quite common, reversible chemical modifications of RNAs molecules which plays a key role in several biological fonctions. This post transcriptional modification can be added by WRITERS, recognized by READERS and also removed byr ERASERS. The METTL3/METTL14 complex plays the role of writer.
| |||||||||||
References
- ↑ . PMID:216315890657
- ↑ . PMID:216315890657
- ↑
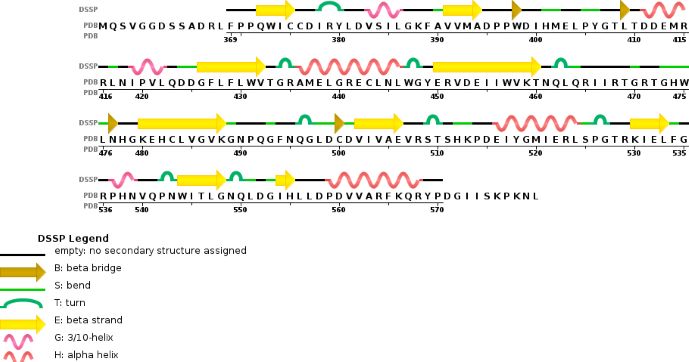
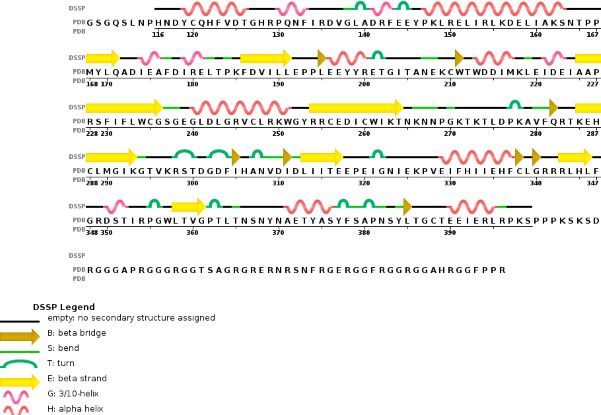
Chain B METTL14 is made of 24% helical s (14 helices; 87 residues) and 18% beta sheet (16 strands; 63 residues)
 <ref>
'''Tertiary structure'''
'''Domains and specific site or sequences'''
Thanks to their primary amino acid sequences both A and B chains have some specific conserved domains directly linked to their function and functionning.
METTL3 and MTTL14 have both a methyltranferase domain but the complex METTL3/METTL14 has a better methyltransferase activity. Moreover, a mutatio in the catalytic center of METTL3 inhibits the hole methyltransferase activity of the complex, whereas a mutation in the catalytic center of METTL14 does not. Thus, METTL3 is the catalytic subunit of the complex and METTL14 enhances the methyltransfearse activity by stabilizing the complex and enables the recognition of consensus sequence on messenger RNA.
[[Image:MET.PNG]]
== Cristal structure ==
To study the crital structure of the METTL3/METTL14 some cristalograghy experiments have been realizes under the following experimental conditions to cristalyze the protein complex:
'''Method''' : Vapor Diffusion Hanging Drop
'''pH '''8
'''Temperature''' : 291.0 K
'''Buffer''': 0.1 M Tris pH 8.0, 20% PEG3350
After critalization, cristal had been analyzed through X-Ray diffraction, at 100°C, with a single wavelength coming out of a synchrotron.
Thanks to the experiment the following data have been collected to describe the cristal structure of the complex :
Unit Cell caracteristics :
'''Length (Å)''' a = 101.86 b = 101.86 c = 117.66
'''Angle (°)''' α = 90 β = 90 γ = 90
'''Symmetry :
Space Group''' P 41 21 2
The primitive cubic symmetry of the unit cell is driven by two 2-fold axis rotation. Moreover, within this space group which is acentric, chiral, and enantiomorphic with one single lattice translations, eight different representative symmetry operations are needed to get the whole unit cell.
[[Image:Cristallo.jpeg]]
The cristal structure analysis at 1,65 angstrom allow to collect precise and detailled informations about the whole structure like the side chains and also the hydrogen bonds. ''(Cristal analysis data at other resolution are available on [http://www.rcsb.org/pdb/explore/materialsAndMethods.do?structureId=5K7M].)''
== Complex METTL3/METTL14 enzymatic working process ==
The enzymatic complex of two proteins is abble to realize methylation reactions thanks to its sequence and specific which allow it to catalyze some perticular reactions at given sites.
The complex METTL3/METTL14 belongs to the writers group, which means that theses two proteins are taking part to the protein subunit able to realize the post transcriptional modification of the RNA within the humans cells.
The METTL3/METTL14 complex catalyze the methylation reaction. [[Image:methylation.png]]
== Function of the modification within the cell ==
Specific, and well controlled methylation of the nucleic acids is essential for proper gene regulation, whatever the studied organism or modification’s type. However the precise molecular role of m6A is unknown, it is knonw that for most mRNAs, m6A modification appear in long exons, near stop codons, and in 3′ UTRs and that this modification could influence mRNA stability, induce RNA conformational changes, modulate protein-RNA interactions, and even modify microRNA processing
One of the most prevalent modifications observed for mRNAs is N6-methyladenosine (m6A). Recent studies have intensely investigated how m6A-modification of RNA contributes to central events in biology. Nonfonctional m6A actors such as writers like METTL3 or METTL14, but also readers, and erasers are oftern linked to problems in self-renewal of stem cells, circadian clock and developmental defects, obesity, synaptic signaling, and cancers.
== Disease and problems triggered by unfunctionnal METTL3/METTL14 complex==
== Relevance ==
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</li>
<li id="cite_note-3">[[#cite_ref-3|↑]] Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016 Jul 21;63(2):306-17. doi: 10.1016/j.molcel.2016.05.041. Epub 2016 , Jun 30. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/27373337 27373337] doi:[http://dx.doi.org/10.1016/j.molcel.2016.05.041 http://dx.doi.org/10.1016/j.molcel.2016.05.041]</li>
<li id="cite_note-4">[[#cite_ref-4|↑]] Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, Zou T, Yin P. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016 May 25;534(7608):575-8. doi: 10.1038/nature18298. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/27281194 27281194] doi:[http://dx.doi.org/10.1038/nature18298 http://dx.doi.org/10.1038/nature18298]</li>
<li id="cite_note-5">[[#cite_ref-5|↑]] Sledz P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 2016 Sep 14;5. pii: e18434. doi: 10.7554/eLife.18434. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/27627798 27627798] doi:[http://dx.doi.org/10.7554/eLife.18434 http://dx.doi.org/10.7554/eLife.18434]</li>
<li id="cite_note-6">[[#cite_ref-6|↑]] Wang X, Huang J, Zou T, Yin P. Human m(6)A writers: Two subunits, 2 roles. RNA Biol. 2017 Mar 4;14(3):300-304. doi: 10.1080/15476286.2017.1282025. Epub 2017, Jan 25. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/28121234 28121234] doi:[http://dx.doi.org/10.1080/15476286.2017.1282025 http://dx.doi.org/10.1080/15476286.2017.1282025]</li></ol></ref>
<ref>
'''Tertiary structure'''
'''Domains and specific site or sequences'''
Thanks to their primary amino acid sequences both A and B chains have some specific conserved domains directly linked to their function and functionning.
METTL3 and MTTL14 have both a methyltranferase domain but the complex METTL3/METTL14 has a better methyltransferase activity. Moreover, a mutatio in the catalytic center of METTL3 inhibits the hole methyltransferase activity of the complex, whereas a mutation in the catalytic center of METTL14 does not. Thus, METTL3 is the catalytic subunit of the complex and METTL14 enhances the methyltransfearse activity by stabilizing the complex and enables the recognition of consensus sequence on messenger RNA.
[[Image:MET.PNG]]
== Cristal structure ==
To study the crital structure of the METTL3/METTL14 some cristalograghy experiments have been realizes under the following experimental conditions to cristalyze the protein complex:
'''Method''' : Vapor Diffusion Hanging Drop
'''pH '''8
'''Temperature''' : 291.0 K
'''Buffer''': 0.1 M Tris pH 8.0, 20% PEG3350
After critalization, cristal had been analyzed through X-Ray diffraction, at 100°C, with a single wavelength coming out of a synchrotron.
Thanks to the experiment the following data have been collected to describe the cristal structure of the complex :
Unit Cell caracteristics :
'''Length (Å)''' a = 101.86 b = 101.86 c = 117.66
'''Angle (°)''' α = 90 β = 90 γ = 90
'''Symmetry :
Space Group''' P 41 21 2
The primitive cubic symmetry of the unit cell is driven by two 2-fold axis rotation. Moreover, within this space group which is acentric, chiral, and enantiomorphic with one single lattice translations, eight different representative symmetry operations are needed to get the whole unit cell.
[[Image:Cristallo.jpeg]]
The cristal structure analysis at 1,65 angstrom allow to collect precise and detailled informations about the whole structure like the side chains and also the hydrogen bonds. ''(Cristal analysis data at other resolution are available on [http://www.rcsb.org/pdb/explore/materialsAndMethods.do?structureId=5K7M].)''
== Complex METTL3/METTL14 enzymatic working process ==
The enzymatic complex of two proteins is abble to realize methylation reactions thanks to its sequence and specific which allow it to catalyze some perticular reactions at given sites.
The complex METTL3/METTL14 belongs to the writers group, which means that theses two proteins are taking part to the protein subunit able to realize the post transcriptional modification of the RNA within the humans cells.
The METTL3/METTL14 complex catalyze the methylation reaction. [[Image:methylation.png]]
== Function of the modification within the cell ==
Specific, and well controlled methylation of the nucleic acids is essential for proper gene regulation, whatever the studied organism or modification’s type. However the precise molecular role of m6A is unknown, it is knonw that for most mRNAs, m6A modification appear in long exons, near stop codons, and in 3′ UTRs and that this modification could influence mRNA stability, induce RNA conformational changes, modulate protein-RNA interactions, and even modify microRNA processing
One of the most prevalent modifications observed for mRNAs is N6-methyladenosine (m6A). Recent studies have intensely investigated how m6A-modification of RNA contributes to central events in biology. Nonfonctional m6A actors such as writers like METTL3 or METTL14, but also readers, and erasers are oftern linked to problems in self-renewal of stem cells, circadian clock and developmental defects, obesity, synaptic signaling, and cancers.
== Disease and problems triggered by unfunctionnal METTL3/METTL14 complex==
== Relevance ==
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</li>
<li id="cite_note-3">[[#cite_ref-3|↑]] Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016 Jul 21;63(2):306-17. doi: 10.1016/j.molcel.2016.05.041. Epub 2016 , Jun 30. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/27373337 27373337] doi:[http://dx.doi.org/10.1016/j.molcel.2016.05.041 http://dx.doi.org/10.1016/j.molcel.2016.05.041]</li>
<li id="cite_note-4">[[#cite_ref-4|↑]] Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, Zou T, Yin P. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016 May 25;534(7608):575-8. doi: 10.1038/nature18298. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/27281194 27281194] doi:[http://dx.doi.org/10.1038/nature18298 http://dx.doi.org/10.1038/nature18298]</li>
<li id="cite_note-5">[[#cite_ref-5|↑]] Sledz P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 2016 Sep 14;5. pii: e18434. doi: 10.7554/eLife.18434. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/27627798 27627798] doi:[http://dx.doi.org/10.7554/eLife.18434 http://dx.doi.org/10.7554/eLife.18434]</li>
<li id="cite_note-6">[[#cite_ref-6|↑]] Wang X, Huang J, Zou T, Yin P. Human m(6)A writers: Two subunits, 2 roles. RNA Biol. 2017 Mar 4;14(3):300-304. doi: 10.1080/15476286.2017.1282025. Epub 2017, Jan 25. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/28121234 28121234] doi:[http://dx.doi.org/10.1080/15476286.2017.1282025 http://dx.doi.org/10.1080/15476286.2017.1282025]</li></ol></ref>