You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Structural highlights of the METTL3/METTL14 complex
[1]
Primary structure
This complex is composed of two differents proteins forming two distinct but linked subunits of the enzymatic complex. Each of these two proteins is coded by one different gene. The mettl3 gene codes for the N6-adenosine-methyltransferase 70 kDa subunit, which is a 225 Amino acid chain, whereas the Mettl14 gene codes for the N6-adenosine-methyltransferase subunit METTL14, which is composed of 349 amino acids.
Each of these two proteins are corresponding to the A [2]or the B chain [3] of the complex and are neccessary to the functionning of the whole complex.
Secondary structure
Both polypeptides METTL3 and METTL14 are able to form some secondary structures thank to interchain and intrachain hydrogen bond formation.
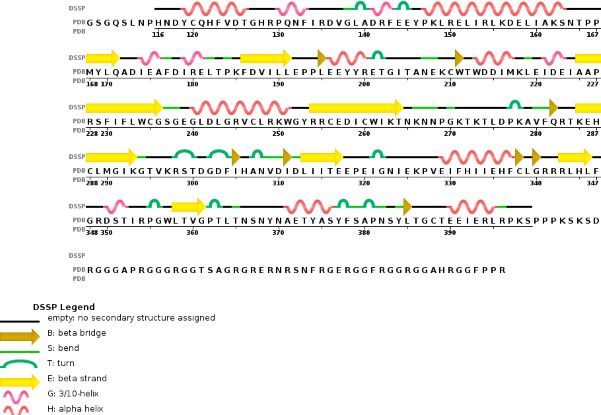
Chain A METTL3'secondary structure, is mainly composed of 20% helical structures (7 helices; 46 residues) and 24% parallel and anti-parallel beta sheets (13 strands; 55 residues)
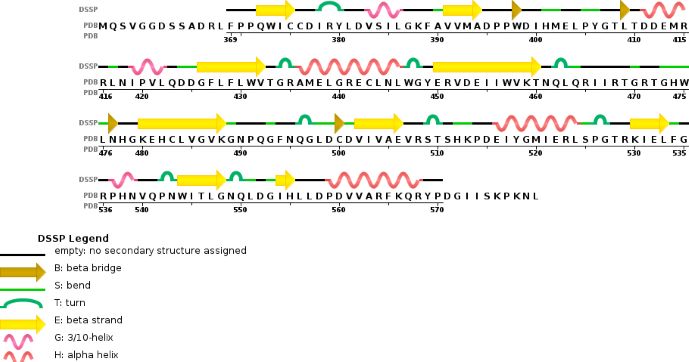
Chain B METTL14 is made of 24% helical structures (14 helices; 87 residues) and 18% parallel and anti-parallel beta sheets (16 strands; 63 residues)
Tertiary structure
Domains and specific site or sequences
Thanks to their primary amino acid sequences both A and B chains have some specific conserved domains directly linked to their function and functionning.
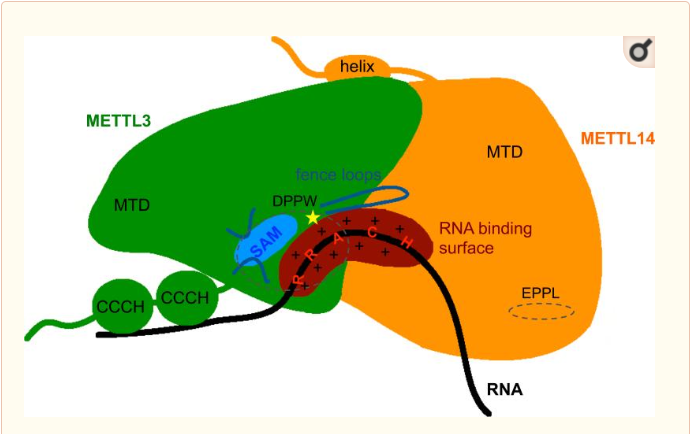
MTD : Methyltransferase domain
METTL3 and MTTL14 have both a methyltranferase domain, which is the domain able to catalyze a methyltransferase reaction.But the complex METTL3/METTL14 has a better methyltransferase activity, than one single subunit activity. Moreover, a mutation in the catalytic center of METTL3 inhibits the hole methyltransferase activity of the complex, whereas a mutation in the catalytic center of METTL14 does not.
Thus, METTL3 is the catalytic subunit of the complex and METTL14 enhances the methyltransferasese activity by stabilizing the complex structure and binding to messenger RNA by enabling the recognition of its consensus sequence.
Zinc finger domain of the METTL3 subunit
Zinc finger domain of the METTL3 N6-methyladenosine methyltransferase, is a RNA binding domain of the complex. This perticular domain of the two proteins complex allow the protein to make interactions with the RNA molecule to modify
Cristal structure
To study the cristal structure of the METTL3/METTL14 some cristalograghy experiments have been realized under the following experimental conditions to cristalyze the protein complex:
Method : Vapor Diffusion Hanging Drop
pH 8
Temperature : 291.0 K
Buffer: 0.1 M Tris pH 8.0, 20% PEG3350
After critalization, cristal had been analyzed through X-Ray diffraction, at 100°C, with a single wavelength coming out of a synchrotron.
Thanks to the experiment the following data have been collected to describe the cristal structure of the complex :
Unit Cell caracteristics :
Length (Å) a = 101.86 b = 101.86 c = 117.66
Angle (°) α = 90 β = 90 γ = 90
Symmetry :
Space Group P 41 21 2
The primitive cubic symmetry of the unit cell is driven by two 2-fold axis rotation. Moreover, within this space group which is acentric, chiral, and enantiomorphic with one single lattice translations, eight different representative symmetry operations are needed to get the whole unit cell.

The cristal structure analysis at 1,65 angstrom allow to collect precise and detailled informations about the whole structure like the side chains and also the hydrogen bonds. (Cristal analysis data at other resolution are available on [4].)
Complex METTL3/METTL14 enzymatic working process
The enzymatic complex of two proteins is abble to realize methylation reactions thanks to its sequence and specific which allow it to catalyze some perticular reactions at given sites.
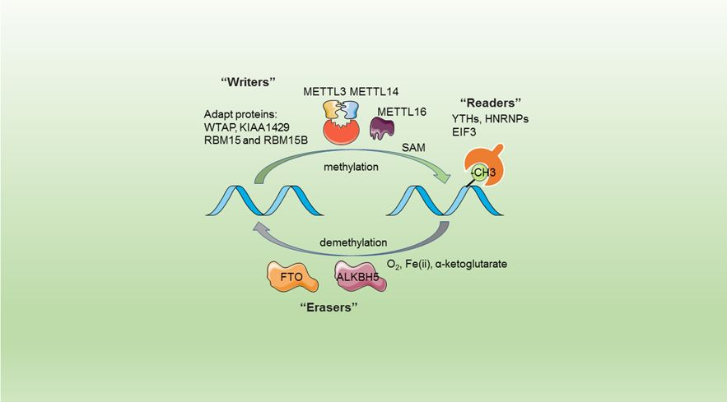
The complex METTL3/METTL14 belongs to the writers group, which means that theses two proteins are taking part to the protein subunit able to realize the post transcriptional modification of the RNA within the humans cells.
The METTL3/METTL14 complex catalyze the methylation reaction. 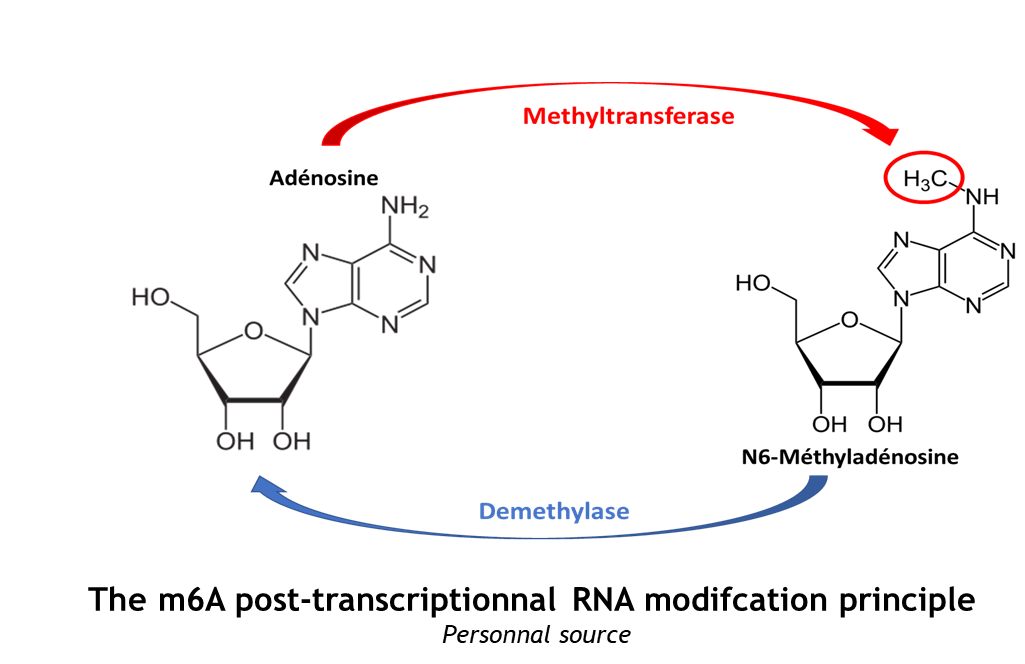
Function of the modification within the cell
Specific, and well controlled methylation of the nucleic acids is essential for proper gene regulation, whatever the studied organism or modification’s type. However the precise molecular role of m6A is unknown, it is knonw that for most mRNAs, m6A modification appear in long exons, near stop codons, and in 3′ UTRs and that this modification could influence mRNA stability, induce RNA conformational changes, modulate protein-RNA interactions, and even modify microRNA processing
One of the most prevalent modifications observed for mRNAs is N6-methyladenosine (m6A). Recent studies have intensely investigated how m6A-modification of RNA contributes to central events in biology. Nonfonctional m6A actors such as writers like METTL3 or METTL14, but also readers, and erasers are oftern linked to problems in self-renewal of stem cells, circadian clock and developmental defects, obesity, synaptic signaling, and cancers.
Disease and problems triggered by unfunctionnal METTL3/METTL14 complex
Relevance
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.