Copper is one of the most important metal involed in multiple enzyme catalysed reactions as a cofactor. In living organisms its function is related to the redox property of the copper. However it is toxic at all concentration, from the lower to the higher, and it need to be strictly controled in living organisms by molecular mechanisms.[1]
Function
Multicopper oxidases are enzymes involved in copper homeostasis. Copper as many metal ions is used in multiple biological processes such as detoxification of oxygen free radicals and pigmentation. However copper present in a cell bot bounded to a protein if harmfull and can cause cellular damage. It need to be regulated.
Multicopper oxidase act probably for the detoxification of copper present in the periplasmic space. It oxidize the Cu+ into Cu2+ and prevent its uptake by the cytoplasm. It also possesses a phenoloxidase and ferroxidase activities which can be involved in the prevention of oxidative damage.[2]
Multicopper oxidase might also be involved in the regulation of metal transport.
Multicopper oxidases are abble to oxdise their substrate. They accept an electron in the and transfer it to the trinuclear copper centre. The dioxygen bind to the trinuclear center and recieve four electron. It is transformed into two molecules of water.[3] Three copper centres exist that can be differentiate spectroscopically: Type 1 or blue (), type 2 or normal (Cu1004) and type 3 or coupled binuclear (1002 and 1003).[4][5]
Mechanism
Multicopper oxidase catalyze the oxidation of different substrates by reducing O2 into H2O without releasing activated oxygen species (H2O2).................
Multicopper oxidase contain 3 types of copper ions involved in the transfer of electrons from the substrate to the dioxygen. The first type of copper, type 1, (Cu1001) mediate the electron transfer fron the substrate to the other copper. The three other copper are called the trinuclear copper center. It is the key element for the oxygen reduction. It contain a type 2 copper (Cu1002) and 2 type 3 copper ions, binuclear ions, (Cu1003 and Cu1004). The final electron acceptor, O2, is bound to this last type of copper and is reduced into two molecules of water.[6]]], </td></tr>
<tr id='related'><td class="sblockLbl">Related:</td><td class="sblockDat">4e9p, 4e9q, 4e9r, 4e9t</td></tr>
<tr id='gene'><td class="sblockLbl">Gene:</td><td class="sblockDat">cueO, yacK, b0123, JW0119 (ECOLI)</td></tr>
<tr id='function'><td class="sblockLbl">Function:</td><td class="sblockDat">binding of a metal ion</td></tr>
<tr id='process'><td class="sblockLbl">Process:</td><td class="sblockDat">oxidation-reduction</td></tr>
<tr id='position'><td class="sblockLbl">Position:</td><td class="sblockDat">bound at the outer membrane in the periplasmic space</td></tr>
<tr id='chain'><td class="sblockLbl">Chain:</td><td class="sblockDat">A</td></tr>
<tr id='sequence domain'><td class="sblockLbl">Sequence domain:</td><td class="sblockDat">Cupredoxin, Multicopper oxidase type 1, type 2, type 3 and a copper-binding site</td></tr>
<tr id='number of amino acids'><td class="sblockLbl">Number of amino acids:</td><td class="sblockDat">489</td></tr>
<tr id='production and extraction'><td class="sblockLbl">Production and extraction:</td><td class="sblockDat">Escherichia Coli</td></tr>
<tr id='initial gene'><td class="sblockLbl">Initial gene:</td><td class="sblockDat">sequence from amino acid 29 to amino acid 516 from P36649</td></tr>
<tr id='specific region'><td class="sblockLbl">Specific region:</td><td class="sblockDat">contain a methionine rich region (aa355 to aa400) that can be important for copper tolerance in bacteria</td></tr>
<tr id='resources'><td class="sblockLbl">Resources:</td><td class="sblockDat">FirstGlance, OCA, PDBe, RCSB, PDBsum, ProSAT, EMBL-EBI</td></tr>
</table>
In the chain, 5 ligands are present: an acetate ion (C2H3O2) and 4 copper ions.
|
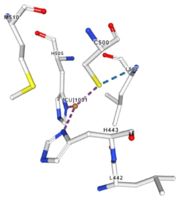
| Cu1001: The copper ion is bound thanks to 3 metal protein interactions with H443, H505 and C500, structure stabilised by L502, M510 by hydrogen bonds |
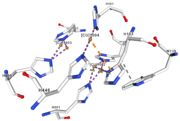
| Cu1002: 2 atoms of Cu, bonds twice by metal interaction (2x3) with 3 histidine : H501, H103, H141 stabilised by hydrophobic contact with W139 |
| Cu1003: 2 CU bond with 3histidine : H499, H143, H448 by 2 metal interactions. h448 is stabilised by Pi interactions with [CU]1004 and H101 |
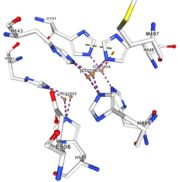
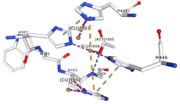 Figure 5: Copper 1004 [10] | Cu1004: one single atome of CU bonds once by metal interactions with 2 H ( and one ACT[1005]) : H101 and H446 stabilised by Pi interactions with H103 and H448. [CU]1004 has Pi interactions with H103 and H448. H113 and H446 Pi interactions |
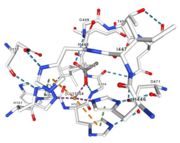 Figure 6: Acetate ion 1005 [11] | ACT[1005]: is linked by hydrogen bonds to G104 and G449 |
Cu1002, Cu1003, Cu1004 and ACT[1005] are near in the space, the 3 CU form a triangle.
The amino acid sequence is:
|
| 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 |
| A | E | R | P | T | L | P | I | P | D | L | L | T | T | D | A | R | N | R | I | Q | L | T | I | G | A | G | Q | S | T |
| 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 |
| F | G | G | K | T | A | T | T | W | G | Y | N | G | N | L | L | G | P | A | V | K | L | Q | R | G | K | A | V | T | V |
| 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 118 |
| D | I | Y | N | Q | L | T | E | E | T | T | L | H | W | H | G | L | E | V | P | G | E | V | D | G | G | P | Q | G | I |
| 119 | 120 | 121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 | 140 | 141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 |
| I | P | P | G | G | K | R | S | V | T | L | N | V | D | Q | P | A | A | T | C | W | F | H | P | H | Q | H | G | K | T |
| 149 | 150 | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 | 161 | 162 | 163 | 164 | 165 | 166 | 167 | 168 | 169 | 170 | 171 | 172 | 173 | 174 | 175 | 176 | 177 | 178 |
| G | R | Q | V | A | M | G | L | A | G | L | V | V | I | E | D | D | E | I | L | K | L | M | L | P | K | Q | W | G | I |
| 179 | 180 | 181 | 182 | 183 | 184 | 185 | 186 | 187 | 188 | 189 | 190 | 191 | 192 | 193 | 194 | 195 | 196 | 197 | 198 | 199 | 200 | 201 | 202 | 203 | 204 | 205 | 206 | 207 | 208 |
| D | D | V | P | V | I | V | Q | D | K | K | F | S | A | D | G | Q | I | D | Y | Q | L | D | V | M | T | A | A | V | G |
| 209 | 210 | 211 | 212 | 213 | 214 | 215 | 216 | 217 | 218 | 219 | 220 | 221 | 222 | 223 | 224 | 225 | 226 | 227 | 228 | 229 | 230 | 231 | 232 | 233 | 234 | 235 | 236 | 237 | 238 |
| W | F | G | D | T | L | L | T | N | G | A | I | Y | P | Q | H | A | A | P | R | G | W | L | R | L | R | L | L | N | G |
| 239 | 240 | 241 | 242 | 243 | 244 | 245 | 246 | 247 | 248 | 249 | 250 | 251 | 252 | 253 | 254 | 255 | 256 | 257 | 258 | 259 | 260 | 261 | 262 | 263 | 264 | 265 | 266 | 267 | 268 |
|
| C | N | A | R | S | L | N | F | A | T | S | D | N | R | P | L | Y | V | I | A | S | D | G | G | L | L | P | E | P | V |
| 269 | 270 | 271 | 272 | 273 | 274 | 275 | 276 | 277 | 278 | 279 | 280 | 281 | 282 | 283 | 284 | 285 | 286 | 287 | 288 | 289 | 290 | 291 | 292 | 293 | 294 | 295 | 296 | 297 | 298 |
| K | V | S | E | L | P | V | L | M | G | E | R | F | E | V | L | V | E | V | N | D | N | K | P | F | D | L | V | T | L |
| 299 | 300 | 301 | 302 | 303 | 304 | 305 | 306 | 307 | 308 | 309 | 310 | 311 | 312 | 313 | 314 | 315 | 316 | 317 | 318 | 319 | 310 | 321 | 322 | 323 | 324 | 325 | 326 | 327 | 328 |
| P | V | S | Q | M | G | M | A | I | A | P | F | D | K | P | H | P | V | M | R | I | Q | P | I | A | I | S | A | S | G |
| 329 | 330 | 331 | 332 | 333 | 334 | 335 | 336 | 337 | 338 | 339 | 340 | 341 | 342 | 343 | 344 | 345 | 346 | 347 | 348 | 349 | 350 | 351 | 352 | 353 | 354 | 355 | 356 | 357 | 358 |
| A | L | P | D | T | L | S | S | L | P | A | L | P | S | L | E | G | L | T | V | R | K | L | Q | L | S | M | D | P | M |
<tr<359 | 360 | 361 | 362 | 363 | 364 | 365 | 366 | 367 | 368 | 369 | 370 | 371 | 372 | 373 | 374 | 375 | 376 | 377 | 378 | 379 | 380 | 381 | 382 | 383 | 384 | 385 | 386 | 387 | 388 | </tr>
| L | D | M | M | G | M | Q | M | L | M | E | K | Y | G | D | Q | A | M | A | G | M | D | H | S | Q | M | M | G | H | M |
| 389 | 390 | 391 | 392 | 393 | 394 | 395 | 396 | 397 | 398 | 399 | 400 | 401 | 402 | 403 | 404 | 405 | 406 | 407 | 408 | 409 | 410 | 411 | 412 | 413 | 414 | 415 | 416 | 417 | 418 |
| G | H | G | N | M | N | H | M | N | H | G | G | K | F | D | F | H | H | A | N | K | I | N | G | Q | A | F | D | M | N |
| 419 | 420 | 421 | 422 | 423 | 424 | 425 | 426 | 427 | 428 | 429 | 430 | 431 | 432 | 433 | 434 | 445 | 436 | 437 | 438 | 439 | 440 | 441 | 442 | 443 | 444 | 445 | 446 | 447 | 448 |
| K | P | M | F | A | A | A | K | G | Q | Y | E | R | W | V | I | S | G | V | G | D | M | M | L | H | P | F | H | I | H |
| 449 | 450 | 451 | 452 | 453 | 454 | 455 | 456 | 457 | 458 | 459 | 460 | 461 | 462 | 463 | 464 | 465 | 466 | 467 | 468 | 469 | 470 | 471 | 472 | 473 | 474 | 475 | 476 | 477 | 478 |
| G | T | Q | F | R | I | L | S | E | N | G | K | P | P | A | A | H | R | A | G | W | K | D | T | V | K | V | E | G | N |
| 479 | 480 | 481 | 482 | 483 | 484 | 485 | 486 | 487 | 488 | 489 | 490 | 491 | 492 | 493 | 494 | 495 | 496 | 497 | 498 | 499 | 500 | 501 | 502 | 503 | 504 | 505 | 506 | 507 | 508 |
| V | S | E | V | L | V | K | F | N | H | D | A | P | K | E | H | A | Y | M | A | H | C | H | L | L | E | H | E | D | T |
| 509 | 510 | 511 | 512 | 513 | 514 | 515 | 516 |
| G | M | M | L | G | F | T | V |
Stabilisation of Cu1001
|
Stabilisation of Cu1002
|
[12]
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.