Sandbox Reserved 1500
From Proteopedia
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
|
Contents |
Structural highlights
| 5hfg is a 1 chain structure. Full crystallographic information is available from OCA. For a guided tour on the structure components use FirstGlance. | |
| Related: | 5hfi |
| Secondary structure: |  Figure 1: 5HFG secondary structure.[1] |
| Resources: | FirstGlance, OCA, PDBe, RCSB, PDBsum, ProSAT |
Global Symmetry: Asymmetric - C1
Global Stoichiometry: Monomer - A
Its theoretical weight is 25.29 KDa
Primary structure
5hfg is a 1 chain structure of 238 amino acids. [2]
Secondary structure
The structure of 5hfg mainly consists in alpha helix (12) , beta sheets (4), you can check the 3D view here. Image:Exemple1.png Image:Exemple2.png
It has 2 main domains, the Lid domain and the thioredoxine domain (see the picture). 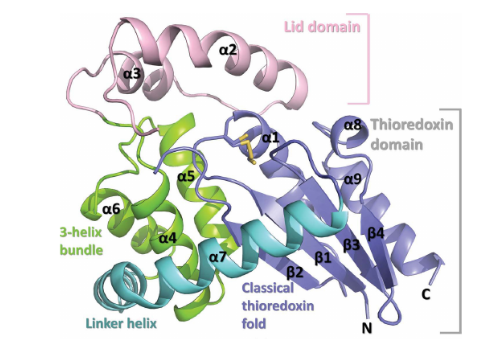
Tertiary structure
5hfg behaves as a monomer or a dimer in solution but it has a monomeric composition. 5hfg is one distinct polypeptide molecule. [3]
Nature
5hfg is a disulfide reductase DsbM from Pseudomonas aeruginosa. The Dsb family of proteins is mainly used to oxidize and reduce cysteine residues of substrate proteins. Most enzymes from Dsb-family catalyze disulfide formation in periplasmic or secreted substrate proteins. This protein contain a CXXC motif which sequence is homologous to the Dsb-family proteins. The CXXC motif is the actif site of the protein for the catalysis of disulfide oxidoreduction, moreover, this site also caracterize the affinity with the substrate protein. [4]
It has no bound ligands and no modified residues. Sequence domains: Thioredoxin-like superfamily DSBA-like thioredoxin domain
Function
This protein has a disulfide oxidoreductase activity, it is implicated in the reduction of the cytoplasmicredox-sensor protein OxyR in Pseudomonas aeruginosa.
Relevance
Click to see a sample scene created with SAT by Group, and to see a transparent representation of the protein.
