Introduction
Histones
Histones are a family of basic, positively charged proteins that associate with DNA inside the nucleus to help condense the DNA into chromatin [1]. The nuclear DNA is wrapped around the histone in order to fit in the nucleus. Nucleosomes are chromatin beads made up of DNA wrapped around eight histone proteins, or a histone octamer [1]. The modification of histones are a type of Epigenetics where changes are made in gene expression without altering the DNA sequence. Four different examples of modifying histones including Histone acetylation, Histone deacetylation, Histone methylation and Histone demethylation [1].
Histone Deacetylases (HDACs)
ε-Amino-lysine acetylation is a type of histone modification that controls the stability of proteins and biological function in eukaryotic cells [2]. Histone Deacetylation is the reversal process for this acetylation modification. There are different classes of HDACs based on phylogenetic analysis:
•Class I - HDACs 1-3 and 8, which are homologous to yeast Rpd3
•Class II - HDACs 4-7, 9 and 10, which are homologous to yeast Hda1
•Class III - Sirtuin deacetylases
•Class IV - HDAC 11 [2].
HDACs 1-11 are metalloenzymes and require a zinc ion for deacetylation [2].
HDAC8
Histone Deacetylase 8 is
Structure
General Structure Information
Inhibitor
Potassium Binding Site
Deacetylation
Zn2+ Metal Ion Mechanism
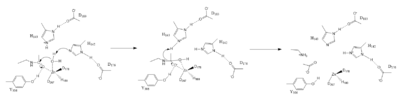
Figure 1. Mechanism of HDAC8
Active Site
Disease
HDACis

