Introduction
Histones are positively charged proteins that help organize DNA into tightly packed chromosomes by acting as a spool for DNA to wrap around. Histones are composed of 4 subunits (H2A, H2B, H3, and H4) and have the capability to loosen or tighten their interactions with DNA to either promote or inhibit transcription. There are a variety of mechanisms that histones achieve these interactions, some examples being the addition or removal of acetyl, methyl, or phosphate groups. These modifications can either increase or decrease the affinity the histone has for the DNA strand. Demethylases are responsible for removing methyl groups from different histone residues. While this is typically associated with increasing histone-DNA interaction, and thus silencing transcription, demethylation has also been associated with the promotion of transcription depending on the residue that is being demethylated.
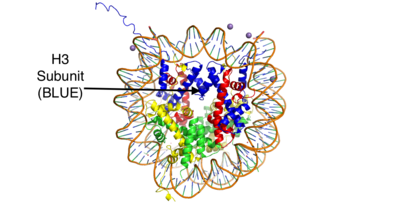
This is the crystal structure of a histone bound to DNA. Its subunits are color coded.
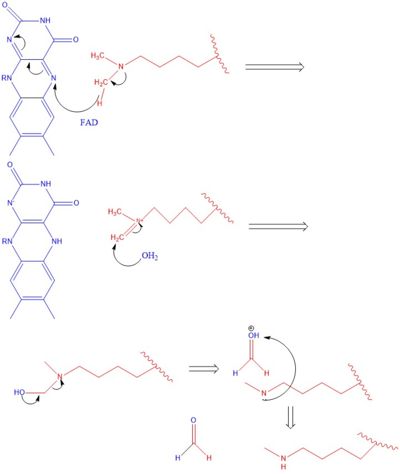
There are two main classes of demethylases, and they are categorized by their co-factors and co-substrates. One class of demethylases uses an FAD co-factor to catalyze the demethylation reaction. The other class of demethylases uses a FE+2 ion and a-ketoglutarate as a co-substrate to catalyze the reaction. Although the co-factors used are different, both classes operate by hydroxylating the target methyl group. Lysine Specific Demethylases 1 is a histone demethylase that uses FAD as a co-factor[1]. Specifically, LSD1 is responsible for demethylating Lys 4 and Lys 9 on the H3 subunit of the histone.
Structure
N-Terminus
Going in order of primary structure, the first ~166 residues are believed to be unstructured and contain a nuclear localization signal. This area of the protein has also been shown to be susceptible to proteolytic cleavage, which may be to remove the localization signal and render protein inactive. However, a mutant of LSD1, which contains residues 166-852 (essentially eliminating the unstructured region) has been shown to be stable and viable when compared to wild-type LSD1 in a photometric activity assay. Unfortunately, this portion of the protein was unable to be crystallized.
SWIRM Domain
The next section of LSD1 spans residues 166-260 and is called the , named after the SWI3, RSC8 and MOIRA proteins from which it was first discovered. It is a highly conserved domain among histone binding proteins, however LSD1's SWIRM domain is unique in that it does not have a positively charged DNA binding domain on the exterior of the protein. Because of this, it believed that LSD1 does not directly bind DNA unlike other histone binding proteins[2][3]. The highly conserved secondary structure of this domain is characterized by a long central helix, with two, shorter helix motifs surrounding it.
Oxidase Domain
The is interesting in that it is not completely continuous in the primary structure. The first portion of this domain spans from residues 280-419 and the second portion of the domain spans from residues 520-852, which is the final residue of the primary protein sequence. Its secondary structure is composed of predominantly of modularity long alpha helices, with a central 4 turn, anti-parallel beta sheet. This is the largest domain of the protein and houses both the active site site and pocket which houses the FAD cofactor.
SWIRM-Oxidase Interface
The interactions between the SWIRM and Oxidase domains create a through a number of hydrophobic (van der Waals) interactions. The interior ends of the helices in the SWIRM domain contribute to the cleft, as well as the alpha helices from the oxidase domains. Because of its vicinity to the active site and FAD co-factor, it is believed that this cleft may serve as a site for additional histone tail binding[4].
Tower Domain
A unique and defining feature of LSD1 is the 100 residue long insertion between the two parts of the oxidase domain in the primary structure. It is known as the and spans from residues 419-520. This domain is unique, yet vital to LSD1 function. Specifically, it is hypothesized to be a binding platform of LSD1 to the CoRest complex, as well as a site of allosteric regulation. The CoRest complex is a group of proteins responsible for the silencing of neuronal genes in non-neural cells, and the binding of LSD1 to this complex activates its demethylase activity. It is composed to two long alpha helices(TaA and TaB) that extend from the core of the protein. The helices hold each other in place through hydrophobic interactions. The TaB helix is the shorter of the two and is connected to a helix in the oxidase domain (aD). aD is essential for active site formation, and TaB is thought to be responsible for the correct of aD[4].
Regulation
Allosteric Stie
LSD-1 is a protein that can be regulated by outside factors. The tower domain and oxidase domain are connected by a . The oxidase domain is what holds the catalytic chamber for LSD-1. This makes the oxidase domain a very sensitive domain. Any change to the catalytic chamber could drastically reduce the ability for the methylated amine to fit in the binding site correctly. Because the tower domain is attached to the oxidase domain, the tower domain and the connector region become allosteric sites. Any interaction with these two sites is hypothesized to drastically change the enzyme activity. This has further pushed the assumption that the CoRest complex interacts with the tower region.[5]
Androgen Receptor
LSD-1 will demethylate mono or di-methylated lysines. However it will not demethylate just any lysine. It will only demethylate lysine H3-K4. This factor can be regulated by the androgen receptor. The androgen receptor is a protein that is involved in DNA transcription. When it interacts with LSD-1 it will no longer demethylate H3-K4, but will now demethylate H3-K9. This attribute allows LSD-1 to work on a wider range of residues.[6]
Mechanism
The methylation of lysine via LSD1 occurs through a 2 electron process. Shown in Figure 3 the first step involves the initiating a hydride transfer from the one of the two methyl groups bound to the nitrogen at the lysine tail, via a N5 on the flavin group. An imine cation is then formed at the end of the lysine tail to compensate for the loss of hydride. In the next step the imine is hydrolyzed and transitions to an Hemiaminal. This then breaks down easily to formaldehyde and the product lysine.
Hydrophobic Pocket
The hydrophobic pocket located in the active site cavity of LSD1, forms a catalytic chamber where the substrate lysine is oriented and positioned to interact with the
FAD co-factor to initiate demethylation. The specific residues making up the pocket include valine-317, glycine-330, alanine-331, methionine 332, valine-333, phenylalanine-338, leucine-569, asparagine-660, lysine-661, tryptophan 695, serine 749, serine 760, and tyrosine-761. The shown in green surrounds the FAD in a way such that it exposes the that is responsible for the two electron demethylation. Another three separate pockets help bind the histone tail residues to the substrate lysine which is essential for identifying different modifications of the histone tail.
Application
LSD1 plays a pivotal role in many biological processes such as cell growth, epithelial-mesenchymal transition, stem cell biology, malignant transformation of cells, and cell differentiation. Malfunctioning of these activities can lead to life threatening diseases such as acute myeloid leukemia and acute lymphoblastic leukemia. Evidence has shown that these activities have ties to prostate and small cell lung cancer so studies have been done to find a reliable inhibitor for LSD1. Monamine oxidases utilize the FAD co-factor much like LSD1 and inhibitors of monamine oxidase have proven to be successful in inhibiting the activity of LSD1. Thus inhibition of LSD1 opens new pathways for possible treatments for cancers and disorders [7].