Functions
See also : Angiotensin_II_receptor_type_1 on Wikipedia
History
Discovery of angiotensin receptors
Researchers suspected since 70s the existence of different angiotensin receptors. However, tools to identify those distinct trans-membrane receptors became available ten years later. Receptors binding assays identified angiotensin receptors in vitro using radioactive angiotensin. Results showed several types of angiotensin receptors, found in different tissues. The main receptors are AT1 and AT2 [1].
Nomenclature
Three labs discovered in the same time these two angiotensin receptors and proposed their own nomenclature, leading to confusion. To avoid this, a group of researchers met in Baltimore in 1991 to define a coherent nomenclature. Under the presidency of Merlin Bumpus, a common ground has been found and angiotensin receptors have been classified into two groups called AT1 and AT2 receptors. [2].
Recent studies
Finally, around 2015, researchers have found the crystal structure of the receptor in complex with its antagonist ZD7155 and with an inverse agonist olmesartan[3]. X-ray cryogenic-crystallography has been used. They have found similar conformation of the receptor when it is linked to the antagonist or to the inverse agonist. They have also found conserved molecular recognition modes. To complete this discovery, they have realized some experiments with mutants to identify the different residues which interact with the ligand.
The structure of this protein have also been solved using an other method called serial femtosecond crystallography, corresponding to the structure 4YAY.
Structure (function relationship)
Primary and secondary structure
Human angiotensin receptor consists in a 376 amino acid string [4]. The protein is composed of and . Moreover, 7 alpha helix are made of a majority of hydrophobic amino acids. These helix are long enough to cross the membrane and create an which is situated into the membrane. The human angiotensin receptor is therefore an alpha helical trans-membrane protein.
Since the angiotensin receptor belongs to the GPCRs family, those 7 alpha helix contain 3 extracellular and 3 intracellular loops.
Ligand binding pocket
In the extracellular environment, there is a beta-hairpin in conjugation with two extracellular disulfure bridges. This structure is responsible for the opening and the locking of the ligand binding pocket [5]. The ligand goes into an created into the membrane thanks to the 7 alpha helix which create a gate between the membrane and the extracellular environment.
G protein-binding site
When the angiotensin II binds to the angiotensin receptor in the ligand binding pocket, the conformation of the trans-membrane domain changes to create a cytosolic cleft for the binding and activation of G proteins. In this cleft, several conserved residues can be found, which form functional motifs present in all GPCRs [6].
Interaction with drugs
Olmesartan anchored to ATR1 by the residues , and .
Those three amino acids seem to play an important role in the binding of the drug to AT1R, thanks to the formation of extensive networks of hydrogen bonds and salt bridges with the ligand [3].
Many drugs used to cure diseases linked with the angiotensin receptor contain a tetrazole group. Studies showed that tetrazole plays an important role in the binding with AT1R.
: an important role for AngII binding
Interaction with other GPCRs
It has been discovered that AT1Rs were also able to bind with other GPCRs to form homo- or heterodimers. Those interactions can modify the sensitivity of the receptor, which leads to different physiological and pathological conditions than the GPCR monomer [3][7]. The most known heterodimers including AT1 receptor are with ß2-adrenergic receptor, the apelin receptor, and AT2 receptor. Those interactions could be facilitated by several transmembrane domains.
The oligomeric complexes' formation complicate the understanding of AT1R pharmacology.
Application in the therapeutic field
Since angiotensin receptor is involved in the renin-angiotenisin system, it represents a target of choice to cure some diseases like hypertension or heart failure.
An over-stimulation of this receptor seems to be involved in hypertension, coronary artery disease, cardiac hypertrophy, heart failure, arrhythmia, stroke, diabetic nephropathy and ischemic heart and renal diseases [7].
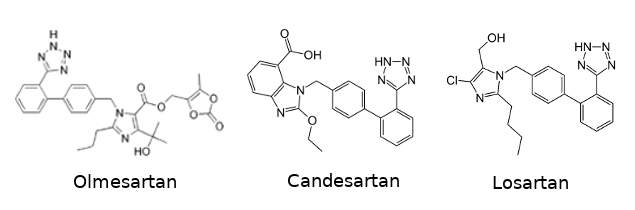
Several anti-hypertensive drugs are targeting the angiotensin receptor in order to block it. This kind of drugs are called angiotensin receptor blockers (ARBs). This category include olmesartan, candesartan, and losartan. One of the common characteristic they share is their biphenyl-tetrazole scaffold.