Function
Zika is a double-stranded RNA virus. The genome replication of double-stranded RNA viruses needs the intervention of a helicase.
Protein from Zika is composed of a protease domain at its N terminus and a helicase domain at its C terminus. The multifunctional helicase belongs to the superfamily 2 (SF2) helicase family. It is this helicase domain that has 5'-triphosphatase activity and that performs the critical and indispensable function of unwinding double-stranded RNA during Zika genome replication. So it is an essential enzyme involved in the cycles of ATP hydrolysis and behaves as a motor protein in the RNA unwinding. Helicase performs ATP hydrolysis at the 5′ end of RNA to generate energy. The phosphorus atom of the γ-phosphate group of the ATP molecule is attacked by a water molecule; then, the γ-phosphate group is released with ADP. With the use of the energy derived from ATP hydrolysis, helicase translocates along nucleic acid strands and unwinds the double-stranded RNA genome.
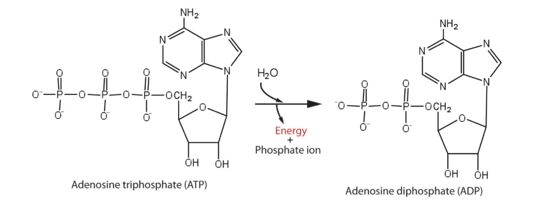
Energy from ATP hydrolysis
This is an essential step for viral RNA replication. It then provides conditions for the polymerization of RNA by an RNA-dependent RNA polymerase and the methylation of RNA by methyltransferase, which is crucial for Zika replication. In addition, it has been reported that the ATPase activity could alter viral replicative capacity and efficiency and consequently change the host innate immune response.
ATP hydrolysis is the most basic event to the function of this helicase; however, the other dynamic mechanisms remain largely unknown, impeding the further understanding of the function of Zika helicase.
[1]
Structural highlights
5y6n is a 1 chain protein of 438 residues.
There are 2 main activities: ATP binding and helicase activity.
Helicase from Zika virus with a specific domain : a serine protease. It is a specific class of protein that hydrolysis peptide bonds from proteins.
The helicase active site has a serine residue that serves as a nucleophilic amino acid. This residue is essential for the catalytic activity of the enzyme.The serine protases present a catalytic triad composed of 3 amino acids His57 Asp102 and Ser195[2].These 3 amino acids have a specific interaction which is due to the folding of the protein. The tridimentional conformation of the protein brings closer these 3 residues. Their side chains have a specific function :

Serine protease catalysis
Ser195: the hydroxyl group plays the role of a nucleophile that attack the substrate.
His57: the nitrogen receives an hydrogen from the hydroxyl group of Ser195.
Asp102: this amino acid allows to increase the electronegativity of His157 nitrogen by making hydrogen bond with His157.
All these reactions allows to cleave the substrate by attacking its carbonyl group.
There are two ligands for zika helicase : ADP and Manganese II ion
-
-
Disease
Zika virus (ZIKV) [3] is a member of the Flavivirus genus, of the virus family Flaviviridae.
The Flavivirus genus comprises the dengue virus (DENV), yellow fever virus, West Nile virus, Japanese encephalitis virus, and tick-borne encephalitis virus. It is transmitted by daytime-active Aedes mosquitoes.
The incubation period (the time from exposure to symptoms) of Zika virus disease is estimated to be 3–14 days.
Only one over five infected people shows mild symptoms like skin rash, fever ("Zika fever"), joint pain, conjunctivitis and less often muscle pain, headache and vomiting. After a few days, the symptoms subside.
An increased risk of neurologic complications is associated with Zika virus infection in adults and children, including Guillain-Barré syndrome, neuropathy and myelitis. Infection during first trimester of pregnancy can cause microcephaly in newborn children.
Like all viruses, Zika virus needs to express itself in its host. The helicase permits the unwinding of the DNA, and finally the virus replication.
It is the non-structural protein 3 (NS3) of the helicase, that is involved in ATP hydrolysis, RNA unwinding and that provides the conditions for RNA polymerization and methylation of RNA which ZIKV needs for replication.
Relevance
There are still no vaccines against Zika virus but the study of zika helicase is one of the most promising area for scientific research. It is an enzyme essential for the virulence of Zika virus and blocking its activity may be a means to struggle against this virus.[4] The development of inhibitors targeting the viral helicase is becoming more and more studied by researchers.
References
- ↑ Mechanism of ATP hydrolysis by the Zika virus helicase, The Faseb journal, 27 sep 2018, X. Yang and H. Yang conceived of and designed the experiments; X. Yang, H. Tian, H. Chi, Z. Mu, and T. Zhang performed the experiments; C. Chen, K. Yang, Q. Zhao, Z. Wang, X. Ji, and H. Yang analyzed the data; and X. Yang, C. Chen, X. Liu, and H. Yang wrote the paper.; https://www.fasebj.org/doi/10.1096/fj.201701140R
- ↑ Enrico Di Cera, Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, Box 8231, St. Louis, MO, USA; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2675663/
- ↑ , "Xiaoyun Yang, Cheng Chen, Hongliang Tian, Heng Chi, Zhongyu Mu, Tianqing Zhang, Kailin Yang, Qi Zhao, Xiaohua Liu, Zefang Wang, Xiaoyun Ji, and Haitao Yang, Mechanism of ATP hydrolysis by the Zika virus helicase, FASEBjournal, pp 5250-5257„https://www.fasebj.org/doi/10.1096/fj.201701140R
- ↑ Xu S, Ci Y, Wang, Yang, Zhang L, Xu C, Qin C, Shi L. Zika virus NS3 is a canonical RNA helicase stimulated by NS5 RNA polymerase.Nucleic Acids Res. 2019 Sep 19;47(16):8693-8707. doi: 10.1093/nar/gkz650.https://www.ncbi.nlm.nih.gov/pubmed/31361901


