scene=
Introduction
selectivity pore
Disease
Relevance
Calcium signaling
Structural highlights and mechanism
The MCU is a dimer of dimers, described as tetrameric truncated pyramid. The uniporter has only a single strong binding site located in the selectivity pore with specificity for
Calcium, near the surface of the inner mitochondrial membrane. Activity of the uniporter is dependent on membrane potential and calcium concentration. Calcium from the cytoplasm enters the mitochondrial innermemnrane space through the mitochondrial membrane and is passed to the mitochondrial matrix via the MCU.
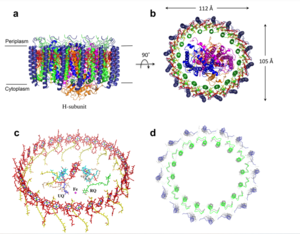
Figure 2: structure of mitochondrial calcium uniporter
Transmembrane Domain
The is on the inner mitochondrial membrane open to the inner membrane space. The small pore, highly specific for calcium binding is located in (TM2) while (TM1) surrounds the pore. The transmembrane domain exhibits four fold rotational symmetry. The domain swapping of TM1 of one subunit with the TM2 of the neighboring subunits allows for a tight packing in the transmembrane connectivity. It is important that the selectivity pore is small, allowing only a dehydrated calcium molecule to interact with the 5 ampier wide glutamate ring. The negative charge of the attracts the positively charged Calcium ion. Approximately one helical turn below the glutamate ring of the selectivity filter, there is a tyrosine ring coming a 12 ampier wide pore allowing high conductivity. The wider opening allows calcium to rehydrate once they pass the selectivity pore. Connectivity between subunits provide flexibility of the uniporter.
Soluble Domain
The is the first subsection of the soluble domain, which resides in the inner mitochondrial membrane. The coiled coil functions as the joints of the uniporter, providing flexibility to promote transport of Calcium ions down their concentration gradient. The junction between the transmembrane domain and the coiled coil's flexibility can be attributed to the disordered packing between subunits; subunits A and C adopt different conformations than the B and D subunits, although they superimpose well.
Image:Symmerty.png Symmetry and organization of subunits from looking down into the uniporter from the inner mitochondrial membrane
When calcium binds to the selectivity pore, the coiled coil swings approximately 8 degrees around its end near the . This movement propagates to the top of the transmembrane domain, where the pore is located, about 85 amperes away. The largest displacement triggered by the movement of the coiled coil is in the transmembrane domain, where the coil bends 20 degrees, moving the transmembrane domain further apart.

