Sandbox Reserved 895
From Proteopedia
| This Sandbox is Reserved from Jan 13 through July 31, 2020 for use in the course Protein Structure in Drug Discovery taught by Karen C. Glass at the ACPHS, Colchester, United States. This reservation includes Sandbox Reserved 895 through Sandbox Reserved 901. |
To get started:
More help: Help:Editing |
1. Introduction
In humans the vertebrate vision is maintained by a chemical process known as the retinoid (visual) cycle. This cycle is a complex enzymatic pathway that operates within the retina to regenerate a key visual chromophore known as 11-cis-retinal. The enzyme responsible for regenerating the key visual chromophore, 11-cis-retinal is a microsomal membrane protein retinal pigment epithelium 65 also known as RPE65. The RPE65 enzyme catalyzes the chemical conversion of all-trans-retinyl ester (or all-trans-retinyl palmityl ester) to 11-cis-retinol within the human retinal pigment epithelium (hRPE). [1]
RPE65 is located within the hRPE cells located in the back of the eye. The human retinal pigment epithelium is responsible for regulating the nourishment of the retina. Since the retinoid (visual) cycle requires the incorporation of light into catalyzing chemical reactions, hRPE cells are light sensitive.
1.1 The Retinoid (Visual) Cycle
| |||||||||||
For humans to see, the retinoid (visual) cycle converts incident light entering into the eye to electrochemical signals that can be transmitted to the brain as neuronal impulses. The chemical reactions that take place within the retinoid cycle allows the regeneration of key intermediates to allow for vision to take place. Dysregulation of these chemical processes, associated with disease, can lead to alterations in vision and blindness. Age related macular degeneration is an example of the dysregulation within the retinoid cycle. The 3-dimensional binding site of bovine RPE65 to an exogenous substrate (R)-emixustat shown in Figure 1, was a tested drug treatment for dry age related macular degeneration.
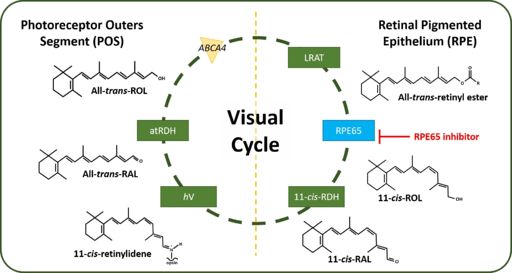
In order to stimulate the phototransduction pathway, 11-cis-retinal must be absorbed into the body. 11-cis-retinal often referred as Vitamin A, is an important dietary vitamin that starts the cascade of phototransduction. Once absorbed into the body and brought to the photoreceptor outer segment (POS), 11-cis-retinal is conjugated to opsin to form the rhodopsin complex. Upon light incidence, 11-cis-retinal is converted though a photo-isomerization reaction into all-trans-retinal. The conversion of 11-cis-retinal to all-trans-retinal causes a conformation change in the rhodopsin complex. This conformation change activates a G-protein coupled protein transducing which triggers the subsequent phototransduction cascade. The generated all-trans-retinal then dissociates from the rhodopsin complex which frees the opsin protein to bind another 11-cis-retinal molecule and restart the phototransduction cascade. All-trans-retinal must be converted back into 11-cis-retinal to serve as the chromophore for the phototransduction cascade. To regenerate 11-cis-retinal a series of enzyme catalyzed chemical reactions must take place. These reactions take place within the photoreceptor outer segment (hPOS) as well as the retinal pigment epithelium (hRPE).
Following dissociation from opsin, all-trans-retinal is transported from the lumen of the photoreceptor disk though an ATP-cassette transporter 4 (ABCA4). The enzyme all-trans-retinol dehydrogenase (atRDH) reduces all-trans-retinal into all-trans-retinol. Another transporter enzyme, interphotoreceptor retinoid-binding protein (IRBP) facilitates the transporter of all-trans-retinol back into the hRPE. Within the hRPE, all-trans-retinol is esterified by the enzyme lecithin retinol acyltransferase (LRAT) to form all-trans-retinyl ester. RPE65 also known as retinoid isomerohydrolase (IMH) then converts all-trans-retinyl ester into 11-cis-retinol and palmityl acid. Following the RPE65 catalyzed reaction, 11-cis-retinol dehydrogenase (11-cis-RDH) oxidizes 11-cis-retinol into 11-cis-retinal. 11-cis-retinal is finally transported back into the photoreceptors to be conjugated to opsin to form the rhodopsin complex. This process is shown in Figure 2. [2]
References
- ↑ Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17325-30. Epub 2009 Oct 5. PMID:19805034
- ↑ Shin Y, Moiseyev G, Petrukhin K, Cioffi CL, Muthuraman P, Takahashi Y, Ma JX. A novel RPE65 inhibitor CU239 suppresses visual cycle and prevents retinal degeneration. Biochim Biophys Acta Mol Basis Dis. 2018 Jul;1864(7):2420-2429. doi:, 10.1016/j.bbadis.2018.04.014. Epub 2018 Apr 21. PMID:29684583 doi:http://dx.doi.org/10.1016/j.bbadis.2018.04.014
- ↑ Shin Y, Moiseyev G, Petrukhin K, Cioffi CL, Muthuraman P, Takahashi Y, Ma JX. A novel RPE65 inhibitor CU239 suppresses visual cycle and prevents retinal degeneration. Biochim Biophys Acta Mol Basis Dis. 2018 Jul;1864(7):2420-2429. doi:, 10.1016/j.bbadis.2018.04.014. Epub 2018 Apr 21. PMID:29684583 doi:http://dx.doi.org/10.1016/j.bbadis.2018.04.014
