Sandbox Reserved 895
From Proteopedia
| This Sandbox is Reserved from Jan 13 through July 31, 2020 for use in the course Protein Structure in Drug Discovery taught by Karen C. Glass at the ACPHS, Colchester, United States. This reservation includes Sandbox Reserved 895 through Sandbox Reserved 901. |
To get started:
More help: Help:Editing |
Contents |
[1] Introduction
| |||||||||||
[1.1] The Retinoid (Visual) Cycle
For humans to see, the retinoid (visual) cycle converts incident light entering into the eye to electrochemical signals that can be transmitted to the brain as neuronal impulses. The chemical reactions that take place within the retinoid cycle allows the regeneration of key intermediates to allow for vision to take place. Dysregulation of these chemical processes, associated with disease, can lead to alterations in vision and blindness. Age related macular degeneration is an example of the dysregulation within the retinoid cycle. The 3-dimensional binding site of bovine RPE65 to an exogenous substrate (R)-emixustat shown in Figure 1A and Figure 1B, was a tested drug treatment for dry age related macular degeneration.
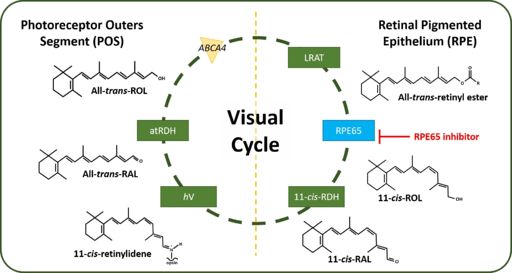
In order to stimulate the phototransduction pathway, 11-cis-retinal must be absorbed into the body. 11-cis-retinal often referred as Vitamin A, is an important dietary vitamin that starts the cascade of phototransduction. Once absorbed into the body and brought to the photoreceptor outer segment (POS), 11-cis-retinal is conjugated to opsin to form the rhodopsin complex. Upon light incidence, 11-cis-retinal is converted though a photo-isomerization reaction into all-trans-retinal. The conversion of 11-cis-retinal to all-trans-retinal causes a conformation change in the rhodopsin complex. This conformation change activates a G-protein coupled protein transducing which triggers the subsequent phototransduction cascade. The generated all-trans-retinal then dissociates from the rhodopsin complex which frees the opsin protein to bind another 11-cis-retinal molecule and restart the phototransduction cascade. All-trans-retinal must be converted back into 11-cis-retinal to serve as the chromophore for the phototransduction cascade. To regenerate 11-cis-retinal a series of enzyme catalyzed chemical reactions must take place. These reactions take place within the photoreceptor outer segment (hPOS) as well as the retinal pigment epithelium (hRPE).
Following dissociation from opsin, all-trans-retinal is transported from the lumen of the photoreceptor disk though an ATP-cassette transporter 4 (ABCA4). The enzyme all-trans-retinol dehydrogenase (atRDH) reduces all-trans-retinal into all-trans-retinol. Another transporter enzyme, interphotoreceptor retinoid-binding protein (IRBP) facilitates the transporter of all-trans-retinol back into the hRPE. Within the hRPE, all-trans-retinol is esterified by the enzyme lecithin retinol acyltransferase (LRAT) to form all-trans-retinyl ester. RPE65 also known as retinoid isomerohydrolase (IMH) then converts all-trans-retinyl ester into 11-cis-retinol and palmityl acid. Following the RPE65 catalyzed reaction, 11-cis-retinol dehydrogenase (11-cis-RDH) oxidizes 11-cis-retinol into 11-cis-retinal. 11-cis-retinal is finally transported back into the photoreceptors to be conjugated to opsin to form the rhodopsin complex. This process is shown in Figure 2. [2]
[1.2] RPE65
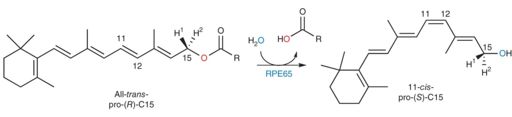
RPE65 is a known retinoid isomerohydrolase (IMH). In the visual cycle RPE65 catalyzes both an isomerization reaction which converts a trans double bond at carbon-11 to a cis configuration as well as a hydrolysis reaction that converts an ester functional group (the retinyl ester) into an alcohol functional group and a carboxylic acid using water. The catalyzed reaction is the rate limiting step in the retinoid cycle. Figure 3 shows the reaction schema. [4]
[1.2.1] Classification of RPE65
According to SCOP, the bovine RPE65 (PDB: 4RSC, SCOP: 8051041), belongs in the domain 8051041, the family 4007172 corresponding to retinoid isomerase RPE65-like, the superfamily 3002594 corresponding to RPE65-like, the fold 2001013 corresponding to 7-bladed beta-propeller and the class 100001 corresponding to all beta proteins. [6]
Using sequence homology, RPE65 belong to a family of proteins known as carotenoid cleavage oxygenase (CCO) enzymes. This class of enzymes often cleave β-carotene or apocarotenoids. However, what makes RPE65 unique form all the other enzymes in this family is that RPE65 catalyzes an isomerhydrolase reaction. Additionally, unlike the other enzymes in the CCO family, there is no obvious role for molecular oxygen in the RPE65 enzymology. All members of the CCO family contain four conserved histidine residues (His180, His241, His313 and His527) that bind to an ion (Fe2+) cofactor. [7] [8]
RPE65 can exist as both soluble and membrane bound forms which can undergo post-translational modifications (PTMs). At cystine residues Cys231, Cys239 and Cys330 the enzyme can be S-palmitoylated. S-palmitoylation of RPE65 was speculated to increase the ratio of membrane-bound to soluble RPE65 which can increase the enzymatic activity. [9] However other studies have challenged this hypothesis and as such palmitoylation of RPE65 require further research to determine the activity. [10]
[1.2.2] Structural Analysis of RPE65
[1.2.2.1] Overall Structural Analysis of RPE65
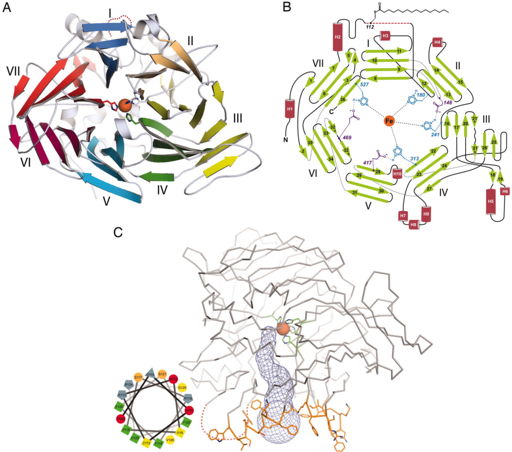
Using the crystal structure of bovine RPE65 (PDB: 3FSN), which is about 99% similar to human RPE65, (although the crystal structure for human RPE65 is not currently available) as the basis of studying the RPE65 structure, RPE65 resembles a seven-bladed β-propeller with single-stranded extension on blades VI and VII and two-stranded extension on blade III shown in Figure 4A. The top face of the β-propeller is defined by connecting the outer strand of the β-sheet with the inner strand of the next β-sheet as shown in Figure 4B. The iron cofactor is located near the top surface of the propeller which is coordinated by four His residues and three secondary Glu residues. Each blade of the propeller contributes one His residue to coordinate with the iron ion. A hydrophobic tunnel leads from the protein exterior to the active site which is defined by the iron ion to accommodate the passage of retinoids (which are conjugated to a fatty acid tail) from the membrane to the RPE65 catalytic site shown in Figure 4C. The mouth of the tunnel is surrounded by three groups of nonpolar residues (169-202, 234-236 and 261-271) that contribute to the overall hydrophobicity of the tunnel and the integration with the lipid bilayer. There are also a few aromatic amino acid side chains that reside in this portion of the enzyme. This suggest that the depth of the RPE65 membrane is restricted to the proximal portions of the phospholipid acyl chains with respect to the polar head groups. Arg and Lys residues within this region also contribute to the association with the negatively charged phospholipid head groups. Using a helical wheel plot, Kiser and colleagues were able to determine that the tunnel region of RPE65 is amphipathic with positive charged residues separating the hydrophobic face from the hydrophilic face as shown in Figure 4C. [11]
[1.2.2.2] Active Site Structural Analysis of RPE65
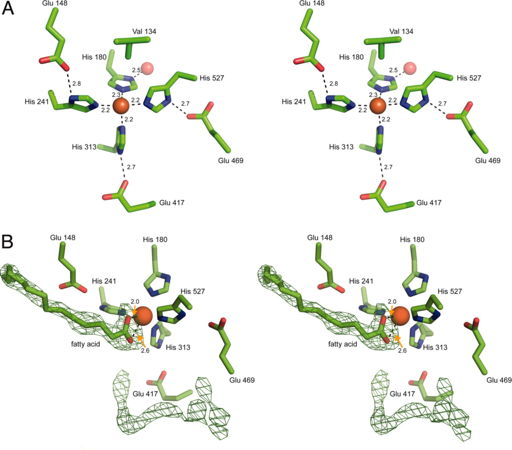
Like stated before the RPE65 iron cofactor is found near the top surface of the β-propeller structure. The iron ion is directly coordinated by the Nitrogen-ε atoms of His180, His241, His313 and His527 with an average bond length of 2.2 angstroms. The geometry of the iron ion coordination is that of an octahedral with two open coordination sties. With the exception of His180, the other coordination interactions (His241, His313 and His527) is via hydrogen bonding from the Nδ-H bond on histidine to the Oε on Glu148, Glu417 and Glu469 respectively. The Nδ-H bond on His180 coordinates with a water molecule that is found lower axial, in the hydrophilic region of the propeller. These hydrogen bonds stabilize the octahedral coordination of the iron ion to facilitate the catalysis reaction. Of the two open coordination sties, one of the sites is occupied by the Cγ atom from Val134 located approximately 4.9 angstroms away. These interactions are shown in Figure 5A.
As stated, the iron ion is also accessible through a main tunnel that enters the helical cap of the protein and is almost orthogonal relative to the propeller axis show in Figure 4C. This tunnel passes though the metal ion forming a bent cavity within the protein. A secondary tunnel within RPE65 leads from the exterior portion of the protein to the active site but contains a narrow segment that occludes the passage of retinoid substrates as well as other substrates catalyzed by RPE65. Although this second tunnel occludes larger compounds, water, small molecules and ions are still permitted into the active site via this tunnel. As such it is hypothesized that the reactants and the products enter and exit from the same tunnel. The presence of amino acid residues such as Phe, Tyr and Trp within the main tunnel help confer enzyme rigidity as well as stabilize the intermediates of the isomerhydrolase reaction. The hydrophobicity of the cavity once again promotes the participation of lipophilic retinoids from the membrane into the active site as well as encourage the reaction of RPE65 with the membrane.
The main tunnel and its interior cavity contain two regions of strong residual electron density that is not accounted for by the atoms located in the protein. This suggest that the substrate would interact with these electron dense regions. Shown in Figure 5B, the first electron dense region is linear with a triangular shape on one end, suggesting the presence of a linear molecule containing a terminal functional group with trigonal planar geometry (such as an ester in the retinyl esters or a carboxylic acid functional group in fatty acids). The triangular portion is in proximity to the iron ion and when positioned correctly fulfills one or both open coordination sites set forth by the octahedral geometry while the linear portion occupies the main tunnel. Also shown in Figure 5B, the second electron dense region is linear and bent in shape which is located within the interior cavity of the protein. This region could be represented by a bound PEG 200 molecule or a string of partially ordered water molecule. However the electron dese region cannot accommodate an longer compound such as (Hydroxyethyloxy)tri(ethyloxy)octane (C8E4).
References
- ↑ Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17325-30. Epub 2009 Oct 5. PMID:19805034
- ↑ Shin Y, Moiseyev G, Petrukhin K, Cioffi CL, Muthuraman P, Takahashi Y, Ma JX. A novel RPE65 inhibitor CU239 suppresses visual cycle and prevents retinal degeneration. Biochim Biophys Acta Mol Basis Dis. 2018 Jul;1864(7):2420-2429. doi:, 10.1016/j.bbadis.2018.04.014. Epub 2018 Apr 21. PMID:29684583 doi:http://dx.doi.org/10.1016/j.bbadis.2018.04.014
- ↑ Shin Y, Moiseyev G, Petrukhin K, Cioffi CL, Muthuraman P, Takahashi Y, Ma JX. A novel RPE65 inhibitor CU239 suppresses visual cycle and prevents retinal degeneration. Biochim Biophys Acta Mol Basis Dis. 2018 Jul;1864(7):2420-2429. doi:, 10.1016/j.bbadis.2018.04.014. Epub 2018 Apr 21. PMID:29684583 doi:http://dx.doi.org/10.1016/j.bbadis.2018.04.014
- ↑ Kiser PD, Zhang J, Badiee M, Li Q, Shi W, Sui X, Golczak M, Tochtrop GP, Palczewski K. Catalytic mechanism of a retinoid isomerase essential for vertebrate vision. Nat Chem Biol. 2015 Jun;11(6):409-15. doi: 10.1038/nchembio.1799. Epub 2015 Apr, 20. PMID:25894083 doi:http://dx.doi.org/10.1038/nchembio.1799
- ↑ Kiser PD, Zhang J, Badiee M, Li Q, Shi W, Sui X, Golczak M, Tochtrop GP, Palczewski K. Catalytic mechanism of a retinoid isomerase essential for vertebrate vision. Nat Chem Biol. 2015 Jun;11(6):409-15. doi: 10.1038/nchembio.1799. Epub 2015 Apr, 20. PMID:25894083 doi:http://dx.doi.org/10.1038/nchembio.1799
- ↑ SCOP Databank 14 Apr 2020 Available from: http://scop.mrc-lmb.cam.ac.uk/term/8051041
- ↑ Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17325-30. Epub 2009 Oct 5. PMID:19805034
- ↑ Shin Y, Moiseyev G, Petrukhin K, Cioffi CL, Muthuraman P, Takahashi Y, Ma JX. A novel RPE65 inhibitor CU239 suppresses visual cycle and prevents retinal degeneration. Biochim Biophys Acta Mol Basis Dis. 2018 Jul;1864(7):2420-2429. doi:, 10.1016/j.bbadis.2018.04.014. Epub 2018 Apr 21. PMID:29684583 doi:http://dx.doi.org/10.1016/j.bbadis.2018.04.014
- ↑ Xue L, Gollapalli DR, Maiti P, Jahng WJ, Rando RR. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell. 2004 Jun 11;117(6):761-71. PMID:15186777 doi:http://dx.doi.org/10.1016/j.cell.2004.05.016
- ↑ Jin M, Yuan Q, Li S, Travis GH. Role of LRAT on the retinoid isomerase activity and membrane association of Rpe65. J Biol Chem. 2007 Jul 20;282(29):20915-24. doi: 10.1074/jbc.M701432200. Epub 2007, May 15. PMID:17504753 doi:http://dx.doi.org/10.1074/jbc.M701432200
- ↑ Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17325-30. Epub 2009 Oct 5. PMID:19805034
- ↑ Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17325-30. Epub 2009 Oct 5. PMID:19805034
- ↑ Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17325-30. Epub 2009 Oct 5. PMID:19805034

