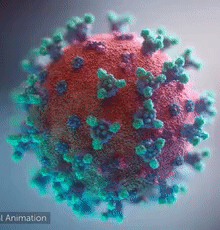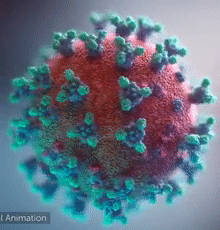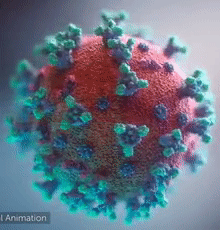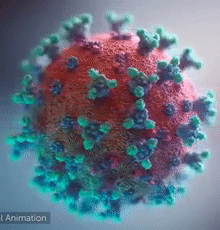
SARS-CoV-2 virus. The spikes, that adorn the virus surface, impart a corona like appearance (Fusion Animation).
A novel coronavirus SARS-CoV-2, detected in Wuhan, China in 2019, was found to cause severe respiratory illness named COVID-19[1].
A video (by Elara Systems) shows how the virus interacts with its human (host) cell, via its spikes (whose 3D structure was determined in the McLellan Lab[2]), thus permitting the viral genome to enter its host & begin infection.
Details of the 3D structure & function of the key proteins & RNA inside the virus can be seen in the NY Times Bad News Wrapped in Protein: Inside the Coronavirus Genome.
Potential treatments for COVID-19
- Oxygen administration could be more important than pressurised air when treating the illness induced by SARS-CoV-2. Homology modeling & molecular docking suggests that SARS-CoV-2 reduces hemoglobin's capacity to carry oxygen, resulting in the respiratory syndrome [3].
- 27-Mar-2020 Science - Not wearing masks to protect against coronavirus is a big mistake, Dr. George Gao, Director General of the Chinese CDC says in Science.
- 23-Mar-2020 A USA, French & UK study identified 69 drugs to test against the coronavirus[4]. As reported in the NY Times.
Videos helping to explain COVID-19
The Czech Republic took the uncommon step of making wearing of masks mandatory in public spaces, prompting a grassroots effort to make masks, with wonderful results
.
Html5mediator: file extension not recognized
Scientists have turned the coronavirus spike protein structure into music(!!), as reported in Science by Soundcloud.
Japan researchers show how Coronavirus spreads through micro droplets. These tiny droplets containing the virus can stay in the air for extended periods.
Speaking face-to-face is exchanging saliva, so stop speaking face-to-face and stay healthy by stoptheviruscovid.
Modeling an epidemic (6-Mar-2020) We're not ready for the next epidemic by 3blue1brown.
Fighting Coronavirus with Soap by PDB-101.
Animation of 3D structure/function of Coronavirus-CoV-2 proteins by biolution GmBH.
COVID-19 outbreak shown via 3D animation (11-Feb 11-2020) by Sci Animations.
Bill Gates Ted Talk (3-Apr-2015) We're not ready for the next epidemic by Ted Talks.
George W. Bush urged us to prepare for future pandemics in 2005, at the NIH.
Jürgen Bosch Presentation (31-Mar-2020) Coronavirus: How it Ticks and What we are Doing to Stop it by Jürgen Bosch.
Useful sites on COVID-19
- Coronavirus Evolved Naturally, and ‘Is Not a Laboratory Construct,’ in a study in Nature Med by Anderson and colleagues [6].
- Scientists are endeavoring to find antivirals specific to the virus. Several drugs such as chloroquine, arbidol, remdesivir & favipiravir are undergoing trials to test their efficacy & safety in the treatment of COVID-19 in China, with some promising results.[7].
- A computer game, developed at the Inst for Protein Design (Univ Washington), is being used to try to find new lead compounds that might become drugs to treat COVID-19.
SARS-CoV-2 virus proteins
The genome of the SARS-CoV-2 virus codes for 28 proteins:
Out of those, 19 have already been characterized structurally.
- Main protease: it is a cysteine protease that is essential for the viral life cycle.
3D structural studies on SARS-CoV-2 virus
- A team of Chinese scientists determined, by Cryo-EM, the coronavirus spike receptor-binding domain complexed with its receptor ACE2 PDB-ID 6LZG. (To be published).
- A team of US and Chinese scientists determined the crystal structure of 2019-nCoV spike receptor-binding domain bound with ACE2 6M0J (To be published).
- A team of US scientists determined, by Cryo-EM, the structure of the SARS-CoV-2 spike glycoprotein (open & closed states) [8], PDB-ID 6VXX & 6VYB
- Crystal structure of SARS-CoV-2 receptor binding domain in complex with human antibody CR3022 6W41 (To be published).
- Crystal Structure of the methyltransferase-stimulatory factor complex of NSP16 and NSP10 from SARS CoV-2 6W61 (To be published).
- Crystal Structure of ADP ribose phosphatase of NSP3 from SARS CoV-2 in complex with AMP 6W6Y (To be published).
- Structure of NSP10 - NSP16 Complex from SARS-CoV-2 6W75 (To be published).
- Crystal structure of SARS-CoV-2 nucleocapsid protein N-terminal RNA binding domain 6M3M (To be published).
- Crystal structure of Nsp9 RNA binding protein of SARS CoV-2 6W4B (To be published).
- Crystal Structure of NSP16 - NSP10 Complex from SARS-CoV-2 6W4H (To be published).
- Cryo-EM structure of the 2019-nCoV RBD/ACE2-B0AT1 complex[9] 6M17.
- Crystal structure of RNA binding domain of nucleocapsid phosphoprotein from SARS coronavirus 2 6VYO (To be published).
- Crystal structure of NSP15 Endoribonuclease from SARS CoV-2 in the Complex with a Citrate 6W01 (To be published).
- Crystal structure of ADP ribose phosphatase of NSP3 from SARS CoV-2 in the complex with ADP ribose 6W02 (To be published).
- Crystal structure of 2019-nCoV chimeric receptor-binding domain complexed with its receptor human ACE2 6VW1 (To be published).
- Crystal structure of NSP15 Endoribonuclease from SARS CoV-2 6VWW (To be published).
- Crystal Structure of ADP ribose phosphatase of NSP3 from SARS CoV-2 6VXS (To be published).
- Crystal structure of the 2019-nCoV HR2 Domain 6LVN (To be published).
- Crystal structure of post fusion core of 2019-nCoV S2 subunit 6LXT (To be published).
- Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors, from the Hilgenfeld lab[10], Apo Struture: PDB-ID 6Y2E, and complexes with inhibitors: PDB-ID 6Y2F and 6Y2G.
- 3D Structure of RNA-dependent RNA polymerase from COVID-19, a major antiviral drug target from the Rao lab in Beijing[11].
- Crystal structure of the Mpro from COVID-19 and discovery of inhibitors in a study by scientists from Shanghai & Beijing[12], PDB-ID 6lu7.
- Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2 in a study by scientists from USA[13], PDB-ID 6w01.
- A study by Zhou & colleagues on the structural basis for the recognition of the SARS-CoV-2 (COVID-19) by full-length human ACE2 gives insights to the molecular basis for coronavirus recognition and infection[14].
- The CoV spike (S) glycoprotein is a key target for vaccines, therapeutic antibodies, and diagnostics. A study by McLellan and colleagues in "Science" on the Cryo-EM structure of the COVID-19 spike protein. This structure should greatly aid in the rapid development and evaluation of medical countermeasures to address the ongoing public health crisis[2], PDB-ID 6vsb.

