[1]
Overview
BRD7 is a human Bromodomain containing protein that is known to be encoded by the BRD7 gene. The full protein has a molecular weight of around 75 kD, while the Bromodomain itself has a molecular weight of roughly 13 kD. It is categorized as a family IV Bromodomain. While it’s function remains largely unidentified, BRD7 is known to recognize Acetylated Lysine residues on the Histones and play role in regulating transcriptional activity. It was first discovered in 2000 in Nasal Pharyngeal Cancer (NPC) cells(1) and has been implicated to have a role in NPC, breast cancer, prostate cancer, and others (2). BRD7 is able to recognize acetyl lysine residues by using its Bromodomain to bind to the Histone tails.
Function
The role of BRD7 in biology is not yet fully understood. It is known however, that BRD7 is part of the PBAF (poly-bromo associated BRG-1 associated factor) complex(3). This is a chromatin remodeling complex that is part of the Swi/Snf family. PBAF is a complex that can facilitate transcription of target genes through chromatin remodeling(4). BRD7 also functions as a tumor suppressor gene that can inhibit p53. It is frequently found to be downregulated in Breast Cancer tumor cells containing wild type p53. BRD7 is important for proper p53 mediated transcription of target genes and is a known p53 co-factor. It likely affects acetylation levels at the promoters of target genes allowing for the facilitation of transcription through regulatino of chromatin dynamics. BRD7 has also been implicated in having function in maintaining male fertility. Although it is still unclear how, BRD7 is involved in spermatogenesis in male mouse models. Knockout of BRD7 during spermatogenesis resulted in complete infertility(5).
Structural Features
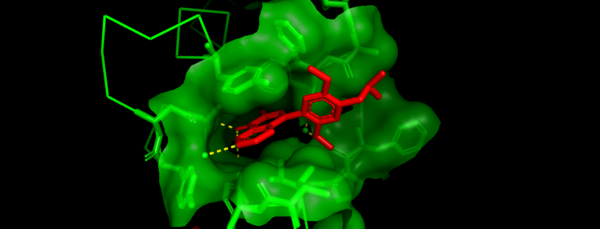
The structure of BRD7 was first solved in 2007 using solution state NMR (6). Like other Bromodomains, BRD7 contains a bundle of 4 left handed antiparallel α-helices. They follow the traditional Bromodomain naming pattern of being called α-Z, α-A, α-B, and α-C in that order from the N-terminal end. In addition to these helices, there are two conserved loops that connect the α-A to the α-Z helix and the α-B to the α-C helix. These are denoted as the ZA and BC loops respectively. The sequence of the BRD7 Bromodomain is shown in Figure 1. The Bromodomain region of BRD7 is contained within the highlighted section and ranges from residues 148-218. To date, two apo structures have been solved for the BRD7 bromodomain using both solution state NMR (6) and X-ray crystallography (7).
Like other related Bromodomains, BRD7 is also known to bind to acetylated Lysines (Kac) on Histones H3 and H4. Specifically, K9 and K14 on H3 (8). BRD7 has also been shown to recognize a variety of Kac restudies on Histone H4 as well. Although there is no known structure of BRD7 in complex with an acetylated Histone peptide, there is in vitro evidence that supports the interactions between BRD7 and Kac (6).
Comparison to other family IV bromodomains
BRD has a similar sequence to other Bromodomains contained within family IV. The other proteins in family IV are: BRD9, BRPF3, BRPF1, BRPF1B, BRD1, ATAD2, and ATAD2B. Shown in Figure 3 is a sequence alignment between BRD7 and its closest related member of family IV, BRD9. The bromodomain sequence is 70 residues long for BRD7 and BRD9. For BRD7, the bromodomain sequence ranges from residue 148-218 while in BRD9 the sequence ranges from 153-223. Despite being the two most closely related bromodomains in family IV, BRD7 and BRD9 only share 71.83% sequence identity.
Implications in disease
BRD7 along with many other Bromodomains has been widely implicated in a variety of diseases like Cancer. There is increasing evidence that BRD7 is highly downregulated in a variety of human cancers. BRD7 is known to act as a crucial component of both the p53 and BRCA1 oncogenic pathways(2). BRD7 is most notably associated with NPC. In NPC cells, it is frequently found that BRD7 is under expressed, which may play a large role in NPC development and progression(9). When BRD7 is overexpressed, it slows the growth of NPC cells through transcriptional regulation.