is a multidomain tumour suppressor protein involved in the regulation of various cellular processes, such as cell adhesion, migration or proliferation[1]. It is expressed in plethora of organs and tissues, e. g. cerebral cortex, bronchi or the gastrointestinal tract[2]. Germline truncation mutations of APC result in familial adenomatous polyposis, a hereditary form of colon cancer[3]. Additionally, loss of the C-terminal portion of APC is detected in about 80 % of sporadic colon cancers[4].
The overall structure of APC
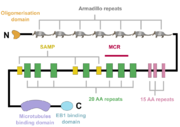
A schematic of the APC protein domain structure. MCR, mutation cluster region; SAMP, Axin-binding motif
The APC protein, its primary sequence encompassing 2843 aminoacids[5], consists of multiple domains, which enable it to interact with diverse partners. At the N-terminus, an oligomerisation domain is found, enabling the APC protein to oligomerise. It is followed by seven so called armadillo repeats, which form a groove for binding of a guanine nucleotide exchange factor Asef[6]. The central part of APC contains three 15 aminoacid long repeats followed by seven 20 aminoacid long repeats[1]. These motifs serve as binding sites for β-catenin[7]. In between the 20 aminoacid repeats, three SAMP regions are dispersed, enabling the interaction with Axin[1]. At the C-terminus, a basic domain responsible for binding to microtubules as well as EB1 interaction domain are present[8][1].
Interestingly, majority of somatic mutations occurs in so called mutation cluster region (MCR) between codons 1286 and 1513 [9].
Disease
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.

