Journal:Acta Cryst D:S2059798320008116
From Proteopedia

Novel structure of the N-terminal helical domain of BibA, a Group B Streptococcus immunogenic bacterial adhesinKartik Manne, Debasish Chattopadhyay, Vaibhav Agarwal, Anna M. Blom, Baldeep Khare, Srinivas Chakravarthy, Chungyu Chang, Hung Ton-That and Sthanam V. L. Narayana [1] Molecular Tour shown as a rainbow model with color changing gradually from the N-terminus (blue) to the C-terminus (red). The antiparallel three-helix-bundle-motif repeats are labeled from the N-terminus to the C-terminus as MR1–MR4, respectively. The overall structure of BibA126–398 has an ~150 Å-long distorted rod shape composed of nine α-helices, a 310-helix and small loops connecting the helices. Interestingly, the nine α-helices adopt a unique pattern to form four antiparallel three-helix-bundle-motif repeats labeled (Asp126–Ser182), (Thr183–Lys235), MR3 (Glu236–Leu303) and MR4 (Gln304–Asp398). Topologically, the N-terminus first assembles into an 18-residue α-helix (α1; Asp126–Ser142) and a small loop (L1; Leu143–Ser147), followed by the second α-helix (α2; Ser148–Ser160), which runs antiparallel to helix α1. Loop 2 (L2; Ser161–Asp163) proceeds α2, allowing the third α-helix (α3; Ser164–Leu195) to run antiparallel to α2. 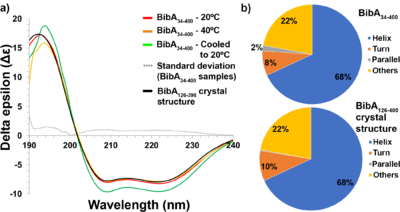 (a) Circular dichroism spectra of freshly prepared and intact BibA34–400 sample at 20°C (red), at 40°C (orange) and denatured at 80°C followed by cooling to 20°C (green), the standard deviation error between the BibA34–400 samples (gray dotted line) and the spectrum generated from the BibA126–398 crystal structure (black). Two negative peaks at 208 and 222 nm typical of a α-helical secondary structure were observed for the BibA34–400 sample. (b) Estimated secondary-structure content (%) of BibA34–400 sample in solution (top) and the spectrum generated from the BibA126–398 crystal structure (bottom). ![(a) Pair distance distribution [P(r)] function of intact BibA34–400 protein. (b) Comparison of the experimental scattering profile (in blue) for BibA34–400 with profiles from a theoretical model (FoXS; green) derived from the proposed BibA34–400 model. (c) Fit of the crystal structure of BibA126–398 (cyan) and the proposed BibA34–400 (magenta) into the ab initio model of BibA34–400 calculated with DAMMIF.](/wiki/images/thumb/9/94/Fig5a-c-relabel-motifs-photoshop-edit.png/500px-Fig5a-c-relabel-motifs-photoshop-edit.png) (a) Pair distance distribution [P(r)] function of intact BibA34–400 protein. (b) Comparison of the experimental scattering profile (in blue) for BibA34–400 with profiles from a theoretical model (FoXS; green) derived from the proposed BibA34–400 model. (c) Fit of the crystal structure of BibA126–398 (cyan) and the proposed BibA34–400 (magenta) into the ab initio model of BibA34–400 calculated with DAMMIF. References
| |||||||||||