From Proteopedia
proteopedia linkproteopedia linkHistone H3.3 |
Introduction
Histone H3.3 is a variant histone of H3 which has the gene name H3.3A and this particular protein is found in Humans. The location of this can be found in the nucleus and in the chromosome.[1]
Function
Histone H3 replaces H3 in active genes and it takes over the original H3 in non dividing cells. The nucleosomes can then wrap around and compact DNA into chromatin and then as a result, it limits DNA access to cellular machineries that would need DNA to serve as a template. It also serves as a replacement histone that's inside chromatin regions by the HIRA chaperone, after the consumption of H3.1 during transcription and DNA repair. They are also needed in regulation of transcription, DNA replication and repair, etc. Access to DNA is regulated by histone code, and nucleosome remodeling.[2]
Disease
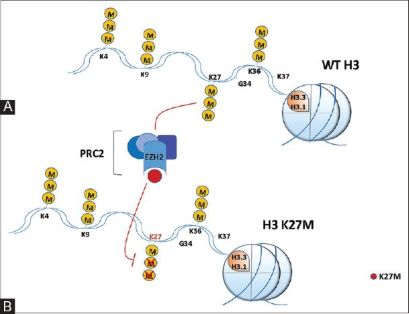
There have been studies that have identified mutations encoding a K27M substitution and there have also been mutations that encoded
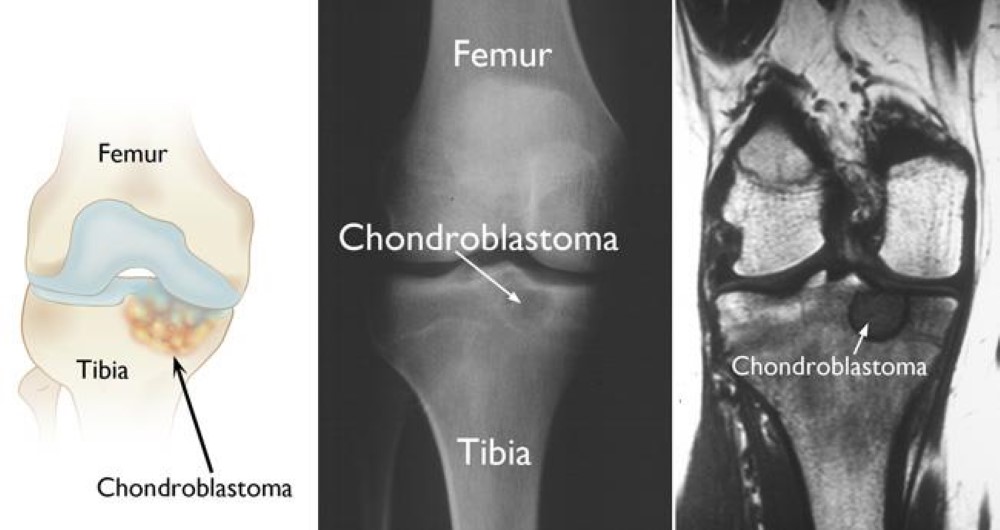
GLY 34 to ARG or VAL called the G34R/V substitution. K27M tumors are spotted in the spinal cord, pons, thalamus for example and G34R/V tumors are shown in the cerebral hemispheres. There are mutations in H3.3 that are found in different types of bone tumors like chrondroblastoma for example and even giant cell tumors of the bone. Chondroblastomacan be seen in children and in young adults (if they are diagnosed with it) in the cartilage of the growth plates of the long bones but most are usually benign.
Glioma is another disease caused by the H3F3A mutation, however they are not harmful or either malignant central nervous system of abnormal growth of tissue from glial cells.
Relevance
Histone octamer contains two of H2A, H2B, H3 and H4 and the octamer wraps approx 147bp of DNA. H3.3 can interact with HIRA and ZMYND11 when trimethylated at (color coded dark blue)[3]. HIRA can deposit histones of H3.3 in replicating and non replicating cells. Also in the HIRA complex, Anti slicing factors like ASF1a and ASF1b are purified by H3.3. However, when there's a disappearance of the HIRA complex, it can cause defects in the early stages of embryogenesis. ZMYND11 also known as BS69 can find H3.3 LYS 36 trimethylation (H3.3K36me3) by the binding domain of chromatin [4].
Structural highlights
CENP-A has a specific centromere and it's controlled in normal cells and its chromosome localization is heavily restricted in the centromere regions. It can be over expressed in cancer cells and can also be abnormally located in the form of heterotypic nucleosomes containing H3.3 [5]. In vitro, the human CENP-A nucleosomes can have two copies each of CENP-A, H2A, H2B and H4 histones [6]. is located on H3.3 and is on the E chain (color coded light pink). The significance to this is that ARG 49 of Asf1 is maintained as either threonine or glutamic acid. Histone H2B located on chains D and H (color coded dark blue), histone H2A type 1-B/E which is located chains C and G (also color coded dark blue),Histone H3-like centromeric protein A is located on chain A (color coded in the lightest blue),and Histone H4 is located on chains B and F (color coded medium blue) [7].
|
References
- ↑ https://www.uniprot.org/uniprot/P84243
- ↑ https://www.uniprot.org/uniprot/P84243
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5446305/
- ↑ https://www.pnas.org/content/112/22/6814
- ↑ https://www.nature.com/articles/srep07115
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3585026/
- ↑ https://www.rcsb.org/structure/3WTP
1. Arimura, Y.; Shirayama, K.; Horikoshi, N.; Fujita, R.; Taguchi, H.; Kagawa, W.; Fukagawa, T.; Almouzni, G.; Kurumizaka, H. Crystal structure and stable property of the cancer-associated heterotypic nucleosome containing CENP-A and H3.3. https://www.nature.com/articles/srep07115 (accessed Nov 1, 2020).
2. Cancer Discovery Science Writers. Histone H3.3 Mutations Are Cancer Type-Specific. https://cancerdiscovery.aacrjournals.org/content/3/12/1329.1 (accessed Nov 14, 2020).
3. Gianno, F.; Antonelli, M.; Ferretti2018, E.; Massimino, M.; Arcella, A.; Giangaspero, F. Pediatric high-grade glioma: A heterogeneous group of neoplasms with different molecular drivers.  (accessed Nov 16, 2020).
(accessed Nov 16, 2020).
4. Kallappagoudar, S.; Yadav, R. K.; Lowe, B. R.; Partridge, J. F. Histone H3 mutations--a special role for H3.3 in tumorigenesis? https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4446520/ (accessed Nov 1, 2020).
5. Morell, N.; Rajani, R. Chondroblastoma - OrthoInfo - AAOS. https://orthoinfo.aaos.org/en/diseases--conditions/chondroblastoma (accessed Nov 16, 2020).
6.panelRuiGuo111LijuanZheng111Juw WonPark2RuituLv1HaoChen1FangfangJiao1WenqiXu1ShirongMu3HongWen45JinsongQiu6ZhentianWang1PengyuanYang1FeizhenWu1JingyiHui3XiangdongFu6XiaobingShi4512Yujiang GenoShi7812YiXing212…YangShi891012, A. links open overlay; RuiGuo111; 1; 11; LijuanZheng111; Juw WonPark2; 2; RuituLv1; HaoChen1; FangfangJiao1; WenqiXu1; ShirongMu3; 3; HongWen45; 4; 5; JinsongQiu6; 6; ZhentianWang1; PengyuanYang1; FeizhenWu1; JingyiHui3; XiangdongFu6; XiaobingShi4512; 12; Yujiang GenoShi7812; 7; 8; YiXing212; YangShi891012; 9; 10; Highlights•BS69/ZMYND11 binds H3.3K36me3 and colocalizes with H3.3K36me3 in gene bodies•BS69 directly interacts with EFTUD2; SummaryBS69 (also called ZMYND11) contains tandemly arranged PHD. BS69/ZMYND11 Reads and Connects Histone H3.3 Lysine 36 Trimethylation-Decorated Chromatin to Regulated Pre-mRNA Processing. https://reader.elsevier.com/reader/sd/pii/S1097276514006777?token=A4FD3B8CDE2F310EA514C66E96DC4489F79C8EA96F6FC878DCD4BFC066FA809C2E83C8A9B57353A53915171AD2491D4C (accessed Nov 16, 2020).
7. UniProt ConsortiumEuropean Bioinformatics InstituteProtein Information ResourceSIB Swiss Institute of Bioinformatics. Histone H3.3. https://www.uniprot.org/uniprot/P84243 (accessed Nov 1, 2020).
8.Yuen, B. T. K.; Knoepfler, P. S. Histone H3.3 mutations: a variant path to cancer. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3882088/ (accessed Nov 16, 2020).
 (accessed Nov 16, 2020).
(accessed Nov 16, 2020).


