Fibrinogen ( factor I) is a plasma glycoprotein in the liver, that is important for proper blood clot formication. Fibrinogen is also synthesized in hepatic tissues.
Fibrinogen is composed of three pairs of polypeptide chains: alpha, beta, and gamma [1]
Function
protease thrombin cleaves fibrinogen into monomers. The formation of monomers, fibrinogen beta, and fibrinogen alpha is insoluble fibrin matrix. fibrin works as a binding or trapping agent in the coagulation process, which creates blood clot.
For small wounds, the polymerized fibrin with platelets forms a hemostatic plug over the wounds site. Fibrin is also anti inflammatory which it protects against IFNG- mediated hemorrhage.
Afibrinogenemic pregnant patients will result in spontaneous miscarriage. Fibrinogen supplemental will allow the patients to sustain the pregnancy, therefore, maternal fibrinogen plays a important role for successful pregnancy.[2]
Disease
Congenital afibrinogenemia (CAFBN)
This is an inherited blood disorder where the blood does not clot normally. This disease is caused when fibrinogen is totally absent . Changing in position Arg-35 when Thrombin cleaves the site of Fibrinopeptide leads to alpha- Dysfibrinogenemias. [3]
Nosebleeds, bleeding from the gums and tongue are commons after a minor trauma for people with this disease. bleedings in the brain and internal organs can occur for affected individuals which can lead to be fatal. However, it's rare. Women with this disease can experience an abnormal heavy menstrual bleeding, they could also have a difficult time carrying a pregnancy, and could result in miscarriages. Newborn with this disease can experience bleeding from the umbilical cord stump after birth. The treatment for this disease includes cryoprecipitate, fibrinogen, and plasma (contains clotting factors). [4]
Amyloidosis 8 (AMYL8)
This is hereditary disease which insoluble amyloid proteins deposits in body tissues and organs. This tends to abnormal protein build-up and leads to damaged organs and deaths. The disease is caused by a mutation fibrinogen alpha chain.[5]
This disease does not show symptoms at first stage. when it gets relatively advanced, the symptoms can be lack of appetite, weight loss, fatigue, weakness, shortness of breath, etc. Due to abnormal protein build-up in body organs, organs can be affected such as heart, liver, kidney, skin, etc. This leads to cardiomyopathy, liver failure, skin rash, nephrotic syndrome. However, nervous system is not affected. There is no treatment for this disease [6].
Congenital Dysfibrinogenemia.
This is either inherited or acquired disorder caused by having abnormal form of fibrinogen. the level of fibrinogen is normal, but the protein doesnt not function properly. This leads to an abnormal blood clot formation; it could be increase or decrease ability to clot. Acquired dysfibrinogenemia is more common and associated with liver disease such as hepatitis, liver tumors.
Most of people with this disease have no symptoms and do not need a treatment. [7]
Relevance
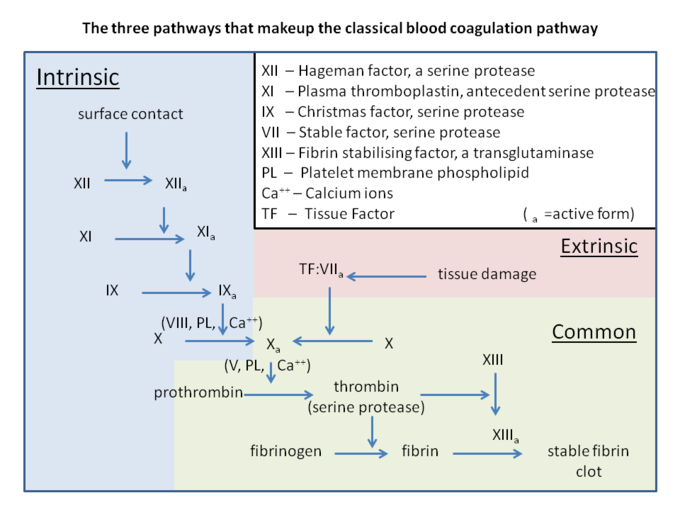
This process called coagulation cascade (or secondary hemostasis) forming a stable insoluble fibrin clot. The process has two pathways which are intrinsic and extrinsic pathway. These pathways eventually join to become the common pathway.
- Intrinsic pathway: This begins with activated factor XII, which then activates factor XI. Activated factor XI further activates factor IX, which activates factor VIII to form tenase complex on a phospholipid surface to activate factor X in the common pathway.
- Extrinsic pathway: when a tissue is damaged, it releases tissue factor to activate factor VII, which then activates factor X.
- Common pathway:'' either intrinsic pathway or extrinsic pathway activates factor X causes formation of thrombin from prothrombin. Thrombin will cleave fibrinogen to insoluble fibrin and also activate factor XIII. Factor XIII and fibrin bind together forming a stable bond and attach to platelets. [8]
Structural highlights
This is the . It is composed of one pairs of alpha, beta, and gamma polypeptides. the polypeptides are oriented to ends meet to form a central E domain which is showed in blue. Both domain on the side of E domain called D domain. The N terms ends of polypeptides are cleaved by thrombin to form gel forming fibrin from soluble fibrin [9].
The black line is , which is an important residue. The mutation of this residue can cause Congenital Dysfibrinogenemia. [10]
This is a structure. [11]

