Hypoxia-inducible factors (HIFs) are transcription factors responsible of the cellular adaptation to hypoxia, which is a condition of low oxygen availability. Among the genes regulated by HIF, we can find those involved in erythropoiesis, angiogenesis and metabolism.
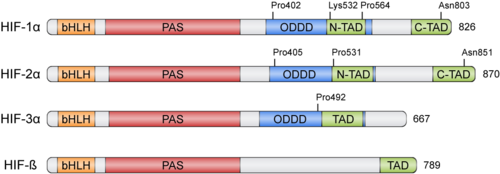
HIFs were discovered more than 30 years ago thanks to the Erythropoietin (EPO) gene, an hormone that is transcribed in hypoxic conditions and stimulates erythrocyte proliferation[3]. EPO presents an upstream Hypoxia Response Element (HRE) that resulted to be bound by HIF [4]. HIFs form part of the basic helix-loop-helix-Per-ARNT-Sim (bHLH-PAS) family of proteins[5]. Active HIFs are achieved by heterodimerization of a constitutively expressed subunit and an oxygen-regulated subunit, which can be HIF-1α or its paralogs and HIF-3α (Table 1.). HIF-β is also known as the aryl hydrocarbon nuclear translocator (ARNT)[6]. Their structure can contain the following domains from the N-terminus to the C-terminus:
- basic Helix-Loop-Helix (): essential for heterodimer formation and DNA binding to HRE
- Per-Arnt-Sim ('): their surface forms the key core of the heterodimer.
- Oxygen-dependent degradation domain (ODDD): mediates oxygen-regulated stability in the α subunits. This domain contains two Proline residues susceptible of hydroxylation and one Lysine that can be acetylated
- N-terminal and C-terminal transactivating domains (N-TAD and C-TAD): bind different coactivators to promote gene expression, such as p300/CBP[7]. HIF-3α lacks one of the TAD domains.
| Member
| Gene
| Protein
|
| HIF-1α | HIF1A | hypoxia-inducible factor 1, α subunit
|
| HIF-2α | EPAS1 | endothelial PAS domain protein 1
|
| HIF-3α | HIF3A | hypoxia-inducible factor 3, α subunit
|
| HIF-1β | ARNT | aryl hydrocarbon receptor nuclear translocator
|
| HIF-2β | ARNT2 | aryl hydrocarbon receptor nuclear translocator 2
|
| HIF-3β | ARNT3 | aryl hydrocarbon receptor nuclear translocator 3
|
Table 1: Members of the HIF family
Physiological Function
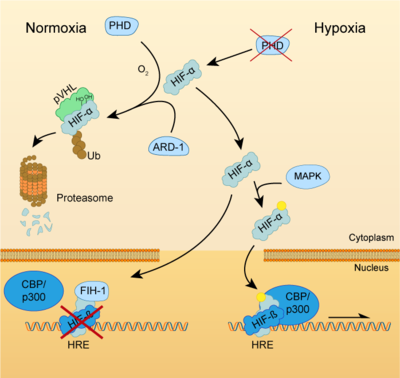
Both HIF-α and β subunits are constitutively and ubiquitously expressed in almost every single cell, although some isoforms may be predominant in some specific tissues[8]. For example, HIF-2α is mostly expressed in the endothelium, kidney, lung, heart and small intestine . While HIF-β protein levels in the cell are constant, HIF-α has a short half-life (~ 5 mins) and is highly regulated by oxygen through its ODDD domain. With normal oxygen levels (normoxia), HIF-α protein levels are rapidly degraded, resulting in essentially no detectable HIF-α protein. However, in hypoxic conditions HIF-α becomes stabilized and is translocated from the cytoplasm to the nucleus, where it dimerizes with HIF-β and the HIF complex formed becomes transcriptionally active. More than 100 genes with varying functions have been described to be transcribed by the action of HIF. Moreover, HIF regulates up to 2% of all human genes in arterial endothelial cells, either directly or indirectly[9]. In response to hypoxia, the capacity of red blood cells to transport oxygen is upregulated by the expression of genes like EPO, essential in erythropoiesis, and genes involved in iron metabolism. In addition, a large number of genes involved in different steps of angiogenesis have been shown to increase by hypoxia challenge, being the Vascular Endothelial Growth Factor (VEGF) among them. VEGF directly recruits endothelial cells into hypoxic areas to generate new blood vessels by stimulating their proliferation. Furthermore, HIF complexes have been shown to induce pro-survival factors such as Insulin-like Growth Factor-2 (IGF2) and Transforming Growth Factor-α (TGF-α), which in turn enhances expression of HIFα itself.
Regulation of HIF-α subunits is achieved by pos-translational modifications such as hydroxylation, ubiquitination, acetylation and phosphorylation. De novo synthesized cytoplasmic HIF-α is rapidly hydroxylated on Pro402 and Pro564 (in HIF-1α) or Pro405 and Pro531 (in HIF-2α) by a family of Prolyl Hydroxylases (PHD) that recognize the consensus sequence LXXLAP . These PHD require an oxygen molecule that will be splitted to catalyze the hydroxylation of the target prolines[10]. Once the two proline residues of HIF-α are converted to hydroxyproline, the Von Hippel-Lindau tumor suppressor (pVHL) will recognize it. pVHL acts as the substrate recognition component of a complex with E3 ubiquitin ligase activity that will then polyubiquitinate HIF-1α on Lysine 532 for proteasomal degradation[11]. In hypoxic conditions, PHDs will be inactive due to the lack of oxygen, so HIF-α can escape proteasomal degradation and translocate to the nucleus to active gene expression. Additional modifications can also modulate HIF-α properties: acetylation of Lys532 by Arrest-Defective-1 (ARD1) favors the interaction of HIF-1α with pVHL[12]; hydroxylation of Asn803 or 851 within the C-CAD domain (in HIF-1α or HIF-2α, respectively) by Factor Inhibiting HIF-1 (FIH-1) prevents interaction with p300/CBP, thus inhibiting transcription[13]. Furthermore, Mitogen-Activated Protein Kinase (MAPK) pathway seems to be able to phosphorylate HIF-α in response to growth factors. This phosphorylation would increase HIF complex transcriptional activity rather than affecting its stability or DNA-binding ability[14].
Clinical Significance
METER FRASE INTRODUCTORIA AQUI
HIF activators
Due to the protective role of HIF, therapies based on small-molecules that stabilize HIF-α have attracted a lot of attention. In pathologies such as ischemia or stroke, HIF activation could help to reduce the damage done by the lack of oxygen and restore tissue functionality. This objective can be achieved by two different ways: inhibition of HIF-PHD pathway or without the inhibition of PHDs [15].
HIF-1α Up-Regulation via Inhibition of PHD Pathway
This group includes the majority of HIF-1α up-regulators. PHDs inhibition can be obtained through different interventions:
Iron chelators and competitors
Iron chelators reduce the number of free iron (Fe2+) by binding tightly to it. In this way, PHD enzymes do not have enough available iron to carry out the hydroxylation reaction and the expression of HIF-1α is upregulated. Also, iron competitors such as Co2+ or Mn2+ can be used [16]. Deferoxamine (DFO) and mimosine were the first iron chelators to mediate HIF-1α neuroprotection. Pre-treatment with DFO protected neurons from oxidative stress-induced death by inhibiting PHDs, therefore increasing mRNA and protein expression of both HIF-1α and its controlled genes [17]. 2,2-dipyridyl (DP) is a liposoluble iron chelator that upregulates HIF-1α expression. Treatment with this drug decreased expansion of tissue damage after ischemia and protected neurons and endothelial cells by decreasing the amount of reactive oxygen species (ROS) [18].
2-oxoglutarate analogs
2-oxoglutarate is also a required co-substrate for the hydroxylation reaction by PHDs. So, compounds such as hydroxybenzenes or their analogous can inhibit PHD action to stabilize HIF-1α an allow gene expression of its targets. Dimethyloxalylglycine
Alternative mechanism of action
Instead of sequestering Fe2+ ions or mimicking