Hypoxia-inducible factors (HIFs) are transcription factors responsible of the cellular adaptation to hypoxia, which is a condition of low oxygen availability. Among the genes regulated by HIF, we can find those involved in erythropoiesis, angiogenesis and metabolism.
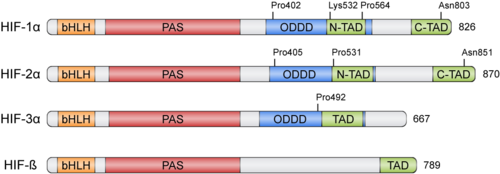
HIFs were discovered more than 30 years ago thanks to the Erythropoietin (EPO) gene, an hormone that is transcribed in hypoxic conditions and stimulates erythrocyte proliferation[3]. EPO presents an upstream Hypoxia Response Element (HRE) that resulted to be bound by HIF [4]. HIFs form part of the basic helix-loop-helix-Per-ARNT-Sim (bHLH-PAS) family of proteins[5]. Active HIFs are achieved by heterodimerization of a constitutively expressed subunit and an oxygen-regulated subunit, which can be HIF-1α or its paralogs and HIF-3α (Table 1.). HIF-β is also known as the aryl hydrocarbon nuclear translocator (ARNT)[6]. Their structure can contain the following domains from the N-terminus to the C-terminus:
- basic Helix-Loop-Helix (): essential for heterodimer formation and DNA binding to HRE
- Per-Arnt-Sim ('): their surface forms the key core of the heterodimer.
- Oxygen-dependent degradation domain (ODDD): mediates oxygen-regulated stability in the α subunits. This domain contains two Proline residues susceptible of hydroxylation and one Lysine that can be acetylated
- N-terminal and C-terminal transactivating domains (N-TAD and C-TAD): bind different coactivators to promote gene expression, such as p300/CBP[7]. HIF-3α lacks one of the TAD domains.
| Member
| Gene
| Protein
|
| HIF-1α | HIF1A | hypoxia-inducible factor 1, α subunit
|
| HIF-2α | EPAS1 | endothelial PAS domain protein 1
|
| HIF-3α | HIF3A | hypoxia-inducible factor 3, α subunit
|
| HIF-1β | ARNT | aryl hydrocarbon receptor nuclear translocator
|
| HIF-2β | ARNT2 | aryl hydrocarbon receptor nuclear translocator 2
|
| HIF-3β | ARNT3 | aryl hydrocarbon receptor nuclear translocator 3
|
Table 1: Members of the HIF family
Physiological Function
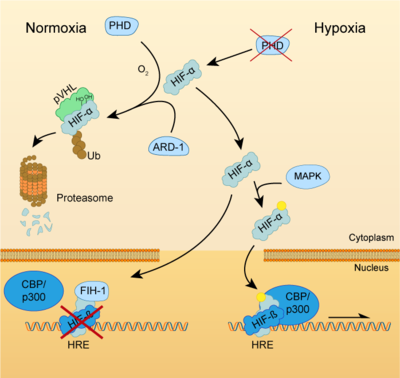
Both HIF-α and β subunits are constitutively and ubiquitously expressed in almost every single cell, although some isoforms may be predominant in some specific tissues[8]. For example, HIF-2α is mostly expressed in the endothelium, kidney, lung, heart and small intestine . While HIF-β protein levels in the cell are constant, HIF-α has a short half-life (~ 5 mins) and is highly regulated by oxygen through its ODDD domain. With normal oxygen levels (normoxia), HIF-α protein levels are rapidly degraded, resulting in essentially no detectable HIF-α protein. However, in hypoxic conditions HIF-α becomes stabilized and is translocated from the cytoplasm to the nucleus, where it dimerizes with HIF-β and the HIF complex formed becomes transcriptionally active. More than 100 genes with varying functions have been described to be transcribed by the action of HIF. Moreover, HIF regulates up to 2% of all human genes in arterial endothelial cells, either directly or indirectly[9]. In response to hypoxia, the capacity of red blood cells to transport oxygen is upregulated by the expression of genes like EPO, essential in erythropoiesis, and genes involved in iron metabolism. In addition, a large number of genes involved in different steps of angiogenesis have been shown to increase by hypoxia challenge, being the Vascular Endothelial Growth Factor (VEGF) among them. VEGF directly recruits endothelial cells into hypoxic areas to generate new blood vessels by stimulating their proliferation. Furthermore, HIF complexes have been shown to induce pro-survival factors such as Insulin-like Growth Factor-2 (IGF2) and Transforming Growth Factor-α (TGF-α), which in turn enhances expression of HIFα itself.
Regulation of HIF-α subunits is achieved by pos-translational modifications such as hydroxylation, ubiquitination, acetylation and phosphorylation. De novo synthesized cytoplasmic HIF-α is rapidly hydroxylated on Pro402 and Pro564 (in HIF-1α) or Pro405 and Pro531 (in HIF-2α) by a family of Prolyl Hydroxylases (PHD) that recognize the consensus sequence LXXLAP . These PHD require an oxygen molecule that will be splitted to catalyze the hydroxylation of the target prolines[10]. Once the two proline residues of HIF-α are converted to hydroxyproline, the Von Hippel-Lindau tumor suppressor (pVHL) will recognize it. pVHL acts as the substrate recognition component of a complex with E3 ubiquitin ligase activity that will then polyubiquitinate HIF-1α on Lysine 532 for proteasomal degradation[11]. In hypoxic conditions, PHDs will be inactive due to the lack of oxygen, so HIF-α can escape proteasomal degradation and translocate to the nucleus to active gene expression. Additional modifications can also modulate HIF-α properties: acetylation of Lys532 by Arrest-Defective-1 (ARD1) favors the interaction of HIF-1α with pVHL[12]; hydroxylation of Asn803 or 851 within the C-CAD domain (in HIF-1α or HIF-2α, respectively) by Factor Inhibiting HIF-1 (FIH-1) prevents interaction with p300/CBP, thus inhibiting transcription[13]. Furthermore, Mitogen-Activated Protein Kinase (MAPK) pathway seems to be able to phosphorylate HIF-α in response to growth factors. This phosphorylation would increase HIF complex transcriptional activity rather than affecting its stability or DNA-binding ability[14].
Clinical Significance
HIFs are involved in many pathologies that involve adaptations to reduced nutrient supply or oxygen availability. In some cases, like in ischemia or stroke, HIF activation can lead to a better cell survival and adaptation, while in pathologies such as cancer, tumoral cells take advantage of HIF properties to enhance their survival inside the body. For this reason, seek of compounds able to modulate HIF activity in both ways has been an active field of research for the past decades.
HIF activators
Due to the protective role of HIF, therapies based on small-molecules that stabilize HIF-α have attracted a lot of attention. In pathologies such as ischemia or stroke, HIF activation could help to reduce the damage done by the lack of oxygen and restore tissue functionality. This objective can be achieved by two different ways: inhibition of HIF-PHD pathway or without the inhibition of PHDs [15].
HIF-1α Upregulation via Inhibition of PHD Pathway
This group includes the majority of HIF-1α upregulators. PHDs inhibition can be achieved through different interventions:
- Iron chelators and competitors: Iron chelators reduce the number of free iron (Fe2+) by binding tightly to it. In this way, PHD enzymes do not have enough available iron to carry out the hydroxylation reaction and the expression of HIF-1α is upregulated. Also, iron competitors such as Co2+ or Mn2+ can be used [15]. Deferoxamine (DFO) and mimosine were the first iron chelators to mediate HIF-1α neuroprotection. Pre-treatment with DFO protected neurons from oxidative stress-induced death by inhibiting PHDs, therefore increasing mRNA and protein expression of both HIF-1α and its controlled genes [16]. 2,2-dipyridyl (DP) is a liposoluble iron chelator that upregulates HIF-1α expression. Treatment with this drug decreased expansion of tissue damage after ischemia and protected neurons and endothelial cells by decreasing the amount of reactive oxygen species (ROS) [17]. Pre-treatment with DP showed more neuroprotection than post-treatment, which makes it difficult to translate into the clinic.
- 2-oxoglutarate: is a required co-substrate for the hydroxylation reaction by PHDs. So, compounds such as hydroxybenzenes or their analogous can inhibit PHD action to stabilize HIF-1α an allow gene expression of its targets. Dimethyloxalylglycine (DMOG) is a cell permeable ester that prevents HIF-1α degradation and increases VEGF levels in ischemic neurons. Studies in animal stroke models showed reduced ischemic injury in neurons and a lower degree of blood-brain barrier damage [18].
- Alternative mechanism of action: Instead of sequestering Fe2+ ions or mimicking 2-oxoglutarate binding, some molecules can bind directly into the active site of PHDs. FG-4497 is a PHD inhibitor that stabilizes HIF-1α and upregulates VEGF and EPO in murine cell lines. It has also been shown to enhance cell survival following oxygen-glucose deprivation. Indeed, intraperitoneal administration of FG-4497 can reduce infarct size[19]. Pre-treatment with this drug also showed neuroprotection following permanent cerebral ischemia. On the other hand, Folic acid (FA) can directly inhibit PHD2, FIH-1 and pVHL. Post-ischemic in vivo treatments showed that FA upregulated HIF-1α and its target genes, conferring neuroprotection[20].
These treatments have some disadvantages because the iron chelators and 2OG analogues can interfere in different pathways. In this sense, some studies have shown the activation of pro-death proteins on brain cells after treatment with PHD inhibitors. So, novel drugs that specifically target HIF-1α would be a better option[15].
HIF-1α Upregulation Without the Inhibition of PHDs
These kinds of molecules have been recently discovered. They can upregulate HIF-1α in different ways:
- MiR-335 is a microRNA that directly regulates HIF-1α. Treatment with this microRNA decreased infarct volume in rat models when administered early after ischemia induction, but inhibition of miR-335 in later phases surprisingly had beneficial effects. This could be due to the biphasic nature of HIF-1α[21].
- Proteasome inhibitors such as MG-132 can stabilize HIF-1α, with better prognosis when combined with DMOG. Other proteasome inhibitors, like epoxomicin or Bsc2118[22], are also able to reduce infarct size and promote cell survival in ischemic conditions, being even more effective than PHDs inhibitors[23].
HIF inhibitors
Given the significant role of the dysregulation of the HIF pathway in several diseases, numerous efforts have been devoted to the development of inhibitors that target the HIF pathway. There are two major categories of the HIF inhibitors: direct HIF inhibitors which affect the expression or function of the HIF molecules, and indirect HIF inhibitors which regulate other molecules in upstream or downstream pathways and affect the transcription, translation or degradation of the factor, ultimately altering the HIF signal as one of the targets.
Indirect HIF inhibitors
Inhibitors of HIF mRNA expression
EZN-2968 is an RNA antagonist composed of a third-generation oligonucleotide, a technology that specifically binds and inhibits the expression of HIF-1α mRNA. It has shown potent (IC50 = 1–5 nM) and selective inhibition of HIF-1α mRNA and protein expression in both normoxia and hypoxia [24]. Preclinical studies both in vitro and in vivo in xenograft prostate cancer mice showed promising results [24]. This compound was evaluated in Phase I clinical trials in patients with advanced solid tumors and was well tolerated [25], while a Phase II trial was inconclusive due to premature closure [26].
Inhibitors of HIF protein translation
- PI3K/Akt/mTOR inhibitors: PI3K/Akt/mTOR plays a major role in the upregulation of HIF-1 several human cancer cell lines, mainly by increasing the rate of HIF-1α protein translation. Although the process by which this pathway regulates HIF protein translation is still poorly understood, several mTOR inhibitors, such as temsirolimus and everolimus, which are two FDA approved agents for the treatment of different types of cancer, have shown to inhibit HIF-1α.
- Camptothecin analogues (CPTs): CPTs analogues are used as chemotherapeutic agents that induce the formation of Topoisomerase I-DNA cleavage complexes, which in the presence of DNA replication generate double strand DNA breaks and cytotoxicity. Among the CPTs is Topotecan, a chemotherapeutic agent that has been approved for the treatment of ovarian cancer, cervical cancer and non-small cell lung carcinoma. This agent act as a HIF inhibitor by blocking HIF-1α protein accumulation and transcriptional activity in a topoisomerase I-dependent manner[27].
- Steroidal HIF inhibitors: 2-Methoxyestradiol (2ME2 or panzem) is a natural metabolite of estradiol that inhibits HIF-1α translation and transcriptional activity. Although the specific mechanism by 2ME2 and its synthetic analogues inhibit HIF is unknown, these compounds bind to the colchicine binding site of tubulin and cause the disruption of tumor interphase microtubules, which results in repression of HIF-1α at translation level [28]. 2ME2 and its synthetic analogues have shown antitumoral activity in preclinical models as well as favorable oral bioavailability, metabolic stability and safety profiles [29]. Unfortunately, 2ME2 has been evaluated in several clinical trials both alone and in combination with other drugs and has shown limited efficacy in the treatment of different types of cancer.
Inhibitors of HIF stabilization
- HSP90 inhibitors: The binding of HSP90 to HIF-1α promotes HIF-1α activity by blocking the pVHL-independent proteasomal degradation and also by helping HIF-1α heterodimers acquire the appropriate conformation to recruit p300 and consequently initiate HIF transactivation. The first HSP90 inhibitor identified was the natural product geldanamycin, a benzoquinone ansamyzine antibiotic that inhibits HSP90 by competing with its ATP binding site. Several HSP90 inhibitors have been developed since, and despite their promising results in preclinical studies, they have demonstrated limited efficacy during clinical trials [30].
- Histone deacetylase inhibitors (HDACi): Histone deacetylases mediate the removal of acetyl groups from target proteins, regulating their function[31]. Treatment with HDACi results in the hyperacetylation of histones and other target proteins such as the chaperone HSP90, leading to a loss of activity. It was recently described that this HSP90 inhibition by an HDACi such as Vorinostat leads to an alteration in HIF-1α signaling by interfering with its ability to translocate to the nucleus[32], although other mechanisms of alteration of HIF-1α signalling by HDAC1 have been proposed[33]. Vorinostat and Romidepsin have been recently approved for the treatment of cutaneous T cell lymphoma (CTCL), and these and others are currently being evaluated for the treatment of various tumors, both alone and in combination with other agents[33].
Direct HIF inhibitors
Inhibitors of HIF-1 dimerization
- PT2385 and PT2399 are recently discovered small molecules and selective HIF-2α inhibitors that bind to the HIF-1β hydrophobic pocket and allosterically block its dimerization with HIF-2α (see more in Cancer).
- Acriflavine is a mixture of trypaflavin (3,6-diamino-10-methylacridinium chloride) and proflavine (3,6-diaminoacridine), which has trypanocidal, antibacterial, and antiviral activities. Acriflavine is also a HIF inhibitor that acts through inhibition of HIF-1 dimerization by binding to the PAS-B subdomain of HIF-1α and HIF-2α[34]. This agent has demonstrated highly promising antitumor activity against a wide spectrum of cancers, including colorectal, hepatocellular, and ovarian cancer cell lines, and more recently in malignant brain cancer both in vitro and in vivo[35]. Its promising safety profile and characteristic ability of binding to both HIF-1α and HIF-2α subunits makes it a promising candidate for clinical evaluation.
Inhibitors of HIF-1 transcriptional activity
- Cardenolides are natural steroids with 25–26 carbon atoms and a five or six-membered lactone ring at C-17. Among them, Digoxin is a widely used cardiac glycoside that has been in clinical use for the treatment of heart failure for many years. This compound also shows HIF-1 inhibitory activity, which has demonstrated efficiency to block tumor growth, invasion, vascularization and metastasis of breast cancer (among other cancer types) both ex vivo and in vivo assays[36]. Digoxin was well tolerated by patients of prostate cancer in a phase II clinical trial and has been evaluated both alone and in combination with other therapies for other types of cancer. It has also demonstrated great efficacy as an inhibitor of pathological angiogenesis in a murine model of ischemic retinopathy[37].
- Chetomin is an antimicrobial dithioketopiperazine fungal metabolite which acts as a HIF-1 inhibitor by blocking its transcriptional co-activation pathway. Chetomin binds to the CH1 domain of the co-activator p300/CBP and disrupts its tertiary structure, thus hindering its interaction with HIF-1 and lowering its transactivation. This compound exhibited antitumoral activity both in vitro and in vivo in a murine model of prostate cancer[38], however, the usefulness of this HIF inhibitor is limited by its toxicity.
- Bortemozib is the first proteasome inhibitor that has been approved by the FDA, in this case for the treatment of multiple myeloma. It has more recently been identified as an HIF-1 inhibitor which simulates the interaction between the C-TAD domain of HIF-1 and FIH target site, thus enhancing FIH mediated repression of p300 recruitment and lowering HIF transactivation[39].
Inhibitors of HIF-1/DNA binding
Echinomycin is a natural cyclic peptide that belongs to the quinoxaline antibiotic family. It also acts as a HIF-1 inhibitor by specifically binding to the core of its recognition sequence, impeding HIF-1 to bind to its target DNA sequence[40]. Although it demonstrated antitumor activity in preclinical studies, no significant antitumoral activity was observed in clinical trials[40].
Stroke
Cancer
In the field of cancer, overexpression and activation of HIF has been associated with malignancy in tumour cells, while low expression has been linked to normal cells and benign tumours. When tumours reach a size of 2-3 mm3, they develop hypoxic regions that, in order to adapt, they overexpress HIF which promote the transcription of several genes involved angiogenesis, cell proliferation, glucose metabolism, metastasis, inflammation, and evasion of anti-tumour immune responses[41]. For instance, HIF-1α helps hypoxic tumour cells to switch their metabolism from oxidative phosphorylation to glycolysis thus triggering in this way the well-known Warburg effect[42]. In fact, intratumoral hypoxia is usually associated with increased risk of invasion, metastasis, and patient mortality.
This way, these factors are directly implicated in the development and progression of several types of cancer. Consequently, HIF regulation is a crucial survival pathway for which novel strategies of cancer therapy could be developed. In fact, a growing number of HIF-1/2 α inhibitors are nowadays being identified, such as PT2385 and PT2977. However, none of them have been clinically approved yet.
PT2385 and PT2977 are, respectively, first and second-generation selective small-molecule inhibitors of HIF-2α, developed for the treatment of advanced ‘’’ccRCC’’’. In this cancer, the pVHL tumor suppressor is defective in approximately 90% of patients, resulting in the stabilization of both HIF-1ɑ and HIF-2ɑ proteins which leads to the expression of a genetic program essential for the initiation and progression of ccRCC (Fig. 1). In fact, it has been demonstrated that HIF-2ɑ is a key tumorigenic driver in ccRCC and, therefore, it constitutes the major cause of malignancy.