This is a default text for your page Madison Unger/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
We used an article on ACAT. We also used an article on SOAT. [3]

Figure 1.Tetrameric dimer of dimer for ACAT
Acyl-coenzyme A: cholesterol acyltransferase (ACAT), also known as Human Sterol O-acyltransferase (hSOAT) is an enzyme that catalyzes the reaction between long chain
fatty acyl CoA and intracellular
cholesterol to form the more hydrophobic cholesteryl ester for cholesterol storage.
Cholesteryl ester is the primary form of how cholesterol is stored in multiple types of cells and transported through the circulatory system. ACAT is an endoplasmic reticulum membrane protein, and ACAT is a part of the
MBOAT (membrane-bound O-acyltransferase) family, which also includes acyl-coenzyme A: diacylglycerol acyltransferase (
DGAT) and ghrelin O-acyltransferase (
GOAT).
There have been two ACAT isoforms discovered in mammals, ACAT1 and ACAT2, and they are predominantly located in different parts of the body. ACAT1 is mainly found in the liver, kidneys, adrenal glands and macrophages, whereas ACAT2 is found only in the intestines and liver.
Structure
Overall Structure
ACAT is a tetramer composed of a dimer of a dimer, but is able to perform its function solely as a . There are in each domain which create a tunnel for the active site. There are also three helices found on the intracellular side (IH1, IH2, and IH3) and one helix on the extracellular side (EH1). The active site contains three tunnels – the transmembrane tunnel for cholesterol entrance, the cytosolic tunnel for acyl-CoA entrance, and the lumen tunnel for cholesterol ester exit. ACAT also has an amino-terminal cytosolic domain (NTD) that is important for tetramerization of this protein.
Important Residues
An important residue in the ACAT active site is His460, a Histidine, which is located in the middle of the tunnels. It is thought that His460 is located on TM7 (Qian et al.). When converting to a cholesteryl ester, the acts as a catalytic base that deprotonates the cholesterol. An asparagine Asn421 is another important residue in the reaction that is able to form a hydrogen bond with acyl-CoA for stabilization.
Important Interactions
Between the two protomers in each dimer, van der waals interactions occur between TM1 of one protomer and the lumenal TM6 and the cytosolic TM9 of the other protomer. The two dimers make limited contact within the membrane through an interface that is thought to be located between TM2, TM5, TM6 and IH2.
Proposed Mechanism
Due to limited high-resolution structural representations of ACAT, its mechanism remains ambiguous. However, the general mechanism involving the substrates and products of ACAT is understood. In this reaction,
oleoyl-CoA and cholesterol are the reactants and they undergo the reaction catalyzed by ACAT to form cholesteryl-oleate which is used as a storage form of cholesterol. The hydroxyl group on cholesterol is deprotonated, then attacks the
thioester bond of oleoyl-CoA, kicking off CoA-SH as a leaving group.
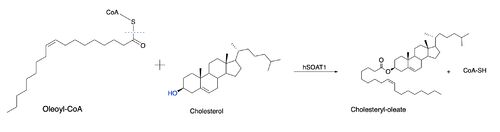
Figure 2: Proposed mechanism for ACAT
However, Qian et al.
[4] proposed a mechanism involving the important residues His460 and Asn421.
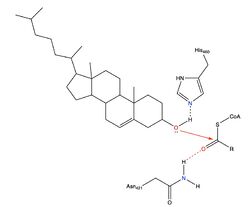
Mechanism for ACAT proposed by Qian et al.
In this mechanism, His460 acts as a general base to deprotonate the hydroxyl group on cholesterol, activating it as a
nucleophile. Then, Asn421 possibly forms a hydrogen bond with oleoyl-CoA to stabilize the reaction.
Active Site
Allosteric Binding Pocket
Tunnels
Disease
Other Protopedia Page
Relevance
Structural highlights
This is a sample scene created with SAT to ">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.[5]



